Changes in Acute Migraine Specific Medications after Initiating





- Slides: 8

Changes in Acute Migraine. Specific Medications after Initiating Erenumab: Results from a Real-World Retrospective Cohort Study in the United States Presentation# EPR 3047 Scan to download a copy of this presentation Dionne M. Hines, 1 Shweta Shah, 2 Jasjit K. Multani, 1 Rolin L. Wade, 1 Dawn C. Buse, 3 Mark Bensink 2 1 IQVIA Inc, Plymouth Meeting, PA, USA; 2 Amgen Inc. , Thousand Oaks, CA, USA; 3 Albert Einstein College of Medicine, Bronx, NY, USA 6 th (Virtual) Congress of the European Academy of Neurology, Paris, 23– 26 May, 2020 Background Methods Results Conclusions

Disclosures Dionne M. Hines: Employee of IQVIA which was contracted by Amgen for this analysis Shweta Shah: Employee and stockholder of Amgen Jasjit K. Multani: Employee of IQVIA which was contracted by Amgen for this analysis Rolin L. Wade: Employee of IQVIA which was contracted by Amgen for this analysis Dawn C. Buse: Research grants from Amgen, consulting fees from Amgen, Allergan, Biohaven, Lilly, Teva, Promeius, and on the speakers bureau for Amgen Mark Bensink: Employee and stockholder of Amgen Funding source: This study was funded by Amgen Inc. Erenumab is codeveloped by Amgen and Novartis Acknowledgment: Medical writing support was provided by Amgen and Novartis. The final responsibility for the content lies with the authors. This e-presentation was supposed to be presented at the 72 nd Annual Scientific Meeting of the American Academy of Neurology, Toronto, Canada, April 25– May 1, 2020. However, due to the COVID-19 pandemic, the physical meeting was cancelled. 2

Hines DM et al. | EPR 3047| Headache and pain 5 Background Retrospective analysis of IQVIA pharmacy claims database 29, 451 patients prescribed ≥ 3 doses of erenumab (index dose: 5/1/2018– 4/30/2019) met inclusion criteria 35% of patients using triptans and 59% of those using ergotamines discontinued their AMSM after starting erenumab (index date) The units of triptans and ergotamines changed in the post-index period in the majority of patients The mean doses of triptan and ergotamine decreased in the post-index period Our analysis provides evidence that erenumab is associated with reductions in AMSM use in real-world clinical practice This retrospective analysis of IQVIA’s open-source pharmacy claims data (LRx) was undertaken to examine real-world changes in AMSM use among patients prescribed erenumab in the US AMSM, acute migraine-specific medications; US, United States 3

Hines DM et al. | EPR 3047| Headache and Pain 5 Methods Study Design • Retrospective, cohort study using IQVIA’s LRx claims database • Patients first prescribed erenumab between May 1, 2018–April 30, 2019 (index date) who were: • − ≥ 18 years of age at the index date − Had an adequate trial of erenumab (ie, ≥ 3 erenumab claims, with ≥ 2 claims within 80 days following the index claim) − Index claim from pharmacy consistently submitting claims to the LRx database (≥ 12 mo pre- and ≥ 6 mo post-index) Descriptive analyses were undertaken LRx, open-source pharmacy claims data 4

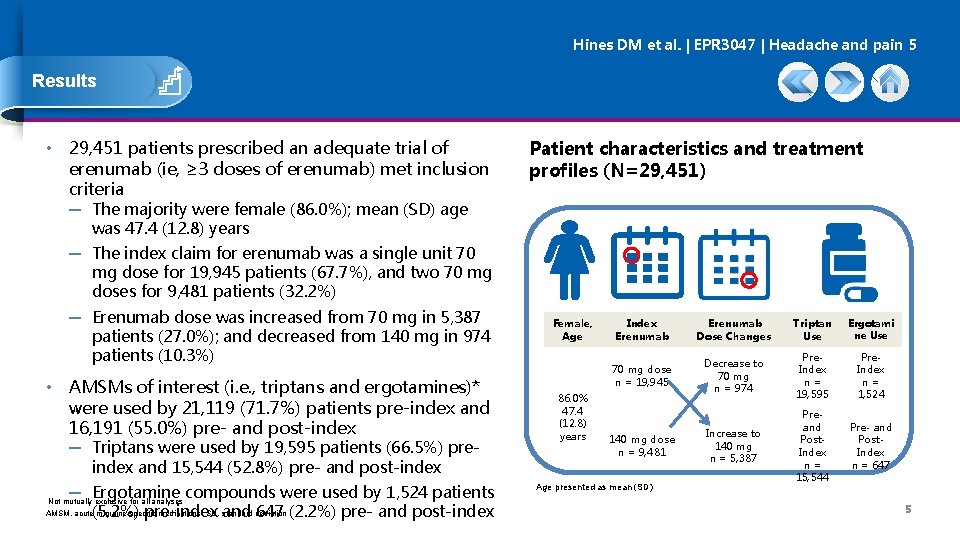
Hines DM et al. | EPR 3047 | Headache and pain 5 Results • 29, 451 patients prescribed an adequate trial of erenumab (ie, ≥ 3 doses of erenumab) met inclusion criteria Patient characteristics and treatment profiles (N=29, 451) ─ The majority were female (86. 0%); mean (SD) age was 47. 4 (12. 8) years ─ The index claim for erenumab was a single unit 70 mg dose for 19, 945 patients (67. 7%), and two 70 mg doses for 9, 481 patients (32. 2%) ─ Erenumab dose was increased from 70 mg in 5, 387 patients (27. 0%); and decreased from 140 mg in 974 patients (10. 3%) • AMSMs of interest (i. e. , triptans and ergotamines)* were used by 21, 119 (71. 7%) patients pre-index and 16, 191 (55. 0%) pre- and post-index ─ Triptans were used by 19, 595 patients (66. 5%) preindex and 15, 544 (52. 8%) pre- and post-index ─ Ergotamine compounds were used by 1, 524 patients Not mutually exclusive for all analyses AMSM, acute(5. 2%) migraine-specific medications; SD, standard pre-index and deviation 647 (2. 2%) pre- and post-index Female, Age 86. 0% 47. 4 (12. 8) years Index Erenumab Dose Changes Triptan Use Ergotami ne Use 70 mg dose n = 19, 945 Decrease to 70 mg n = 974 Pre. Index n= 19, 595 Pre. Index n= 1, 524 Increase to 140 mg n = 5, 387 Preand Post. Index n= 15, 544 Pre- and Post. Index n = 647 140 mg dose n = 9, 481 Age presented as mean (SD) 5

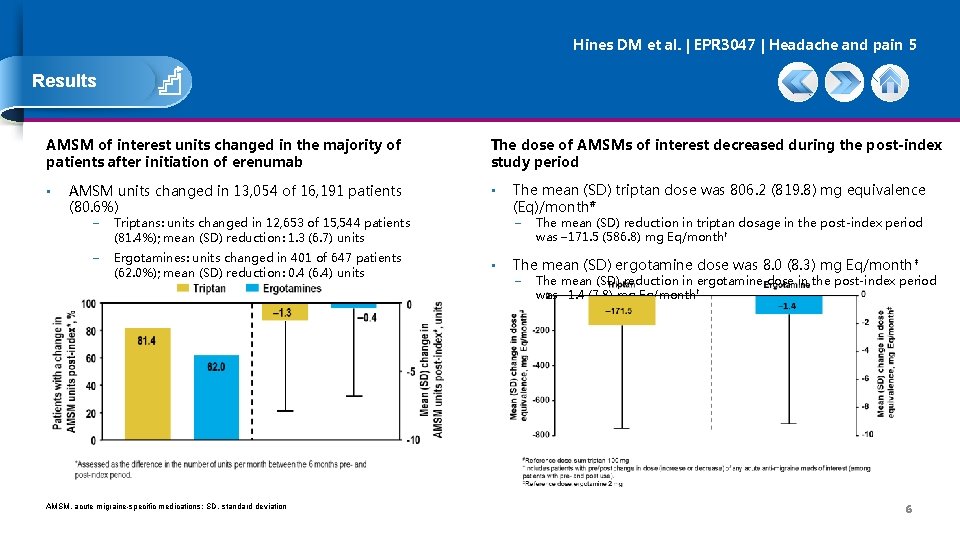
Hines DM et al. | EPR 3047 | Headache and pain 5 Results AMSM of interest units changed in the majority of patients after initiation of erenumab • AMSM units changed in 13, 054 of 16, 191 patients (80. 6%) − Triptans: units changed in 12, 653 of 15, 544 patients (81. 4%); mean (SD) reduction: 1. 3 (6. 7) units − Ergotamines: units changed in 401 of 647 patients (62. 0%); mean (SD) reduction: 0. 4 (6. 4) units AMSM, acute migraine-specific medications; SD, standard deviation The dose of AMSMs of interest decreased during the post-index study period • The mean (SD) triptan dose was 806. 2 (819. 8) mg equivalence (Eq)/month# − • The mean (SD) reduction in triptan dosage in the post-index period was – 171. 5 (586. 8) mg Eq/month† The mean (SD) ergotamine dose was 8. 0 (8. 3) mg Eq/month ‡ − The mean (SD) reduction in ergotamine dose in the post-index period was – 1. 4 (7. 8) mg Eq/month† 6

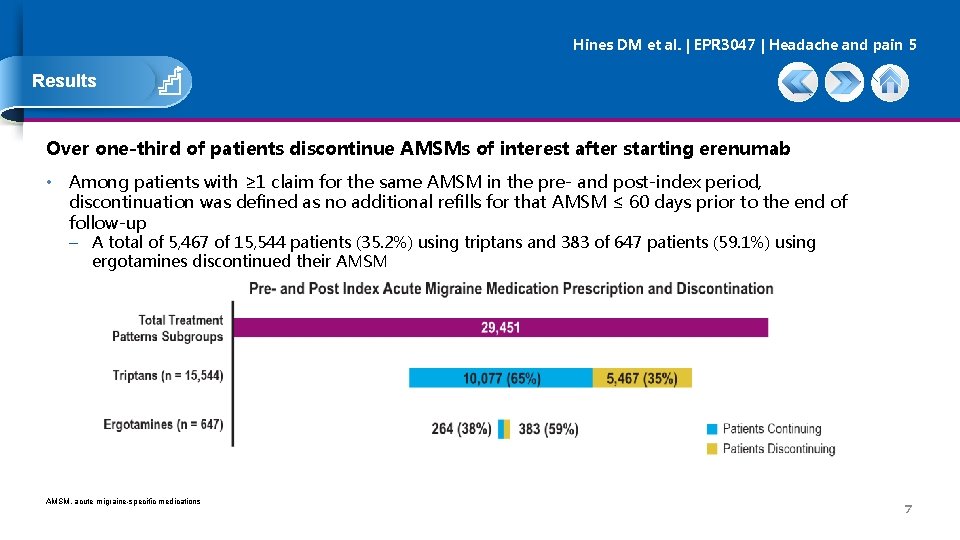
Hines DM et al. | EPR 3047 | Headache and pain 5 Results Over one-third of patients discontinue AMSMs of interest after starting erenumab • Among patients with ≥ 1 claim for the same AMSM in the pre- and post-index period, discontinuation was defined as no additional refills for that AMSM ≤ 60 days prior to the end of follow-up – A total of 5, 467 of 15, 544 patients (35. 2%) using triptans and 383 of 647 patients (59. 1%) using ergotamines discontinued their AMSM, acute migraine-specific medications 7

Hines DM et al. | EPR 3047 | Headache and pain 5 Conclusions • In this US-focused real-world study, 35% of patients using triptans and 59% using ergotamines discontinued these medication after starting erenumab • Among patients continuing to use therapies, mean doses of both medications decreased after staring erenumab • Our analysis provides evidence that the benefits of erenumab seen in clinical trials, in terms of reductions in specific acute migraine medications, are being realised in real-world clinical practice • More research is needed to better understand how significant the reduction in migraine medication burden may be US, United States 8