Cauvery College for Women Autonomous Nationally Accredited III



















- Slides: 22

Cauvery College for Women (Autonomous) Nationally Accredited (III Cycle) with ‘A’ Grade by NAAC Annamalai Nagar, Tiruchirappalli-18.

Name of the Faculty : Dr. H. ABIRAMI Designation : DEAN OF SCIENCE Department : BIOTECHNOLOGY Programme : B. Sc. , BIOTECHNOLOGY Batch : 2016 -2017 Onwards Semester : VI Course : IMMUNOTECHNOLOGY Course Code : 16 SMBEBT 3 Unit : V Topics Covered : VACCINES

PREFAC What is a. E vaccine? Properties of good vaccine • • • Types of vaccines • Advantages of recombinant vaccines over conventional vaccines • Recombinant virus vaccines or live recombinant vaccines • Future Potential • References

WHAT IS A VACCINE? A preparation of killed or weakened microorganism that is given to a person orally or injected in order to prevent disease. • Edward Jenner demonstrated that a person inoculated into the skin with cowpox was protected against small pox and he thus developed the principles of vaccination in 1796. • In 1881 Louis Pasteur honored Jenner by naming the processing ‘’vaccination’’ and the substance used to vaccinate was a ‘’vaccine’’.

• Principle of a vaccine is to induce a primary response in the vaccinated subject so that following the exposure of a pathogen, a rapid secondary immune response is generated leading to accelerated elimination of the organism and protection from clinical disease. • Success depends on the generation of memory T cells and B cells and presence of neutralizing antibody serum.

Properties of a good vaccine • Ability to elicit the appropriate immune response for the particular pathogen. • Long term protection • Safety • Stability • Inexpensiveness

Types of vaccines • Live vaccines • Killed or whole organism vaccines • Subunit vaccines-purified or recombinant antigen • Recombinant vaccines • DNA vaccines

• • • These vaccines are prepared from attenuated strains that are almost or completely devoid of pathogenicity but are capable of inducing a protective immune response to the body. They multiply in human host and provide continuous antigenic stimulation over a period of time. For example typhoid vaccines.

Killed whole organism vaccines • It is a vaccine that is produced by growing the organism and then killing or inactivating it with heat and/or chemicals. • These are used when safe live vaccines are not available • For example inactivated polio vaccine • Rabies vaccine

Subunit vaccines are defined as those vaccines containing one or more pure or semi-pure antigen. These are of three types, toxoids, recombinant subunit vaccines and non recombinant subunit vaccines.

• Toxoid s In some diseases like diphtheria and tetanus it is not the growth of the bacterium that is dangerous, but the protein toxin that is liberated by it. • Treating the toxin with formaldehyde denatures the protein so that it is no longer dangerous. • The inactivated toxin is called as toxoid. • For example, DPT vaccine also called as triple vaccine.

• SUBUNIT VACCINES (NONRECOMBINANT) • Constituent proteins of bacteria or virus are isolated and purified • Advantages: • Defined Composition • Various delivery systems available • Disadvantages: • Antigens must be produced and purified by cultivation of a pathogen • Multiple doses typically required

Subunit recombinant vaccines These vaccines are those in which genes for desired antigens are inserted into a vector, usually a virus, that has a very low virulence. The vector expressing the antigen may be used as the vaccine, or the antigen may be purified and injected as a subunit vaccine. The only recombinant vaccine currently in use in humans is the Hepatitis B Virus (HBV) vaccine, which is a recombinant subunit vaccine Hepatitis B surface antigen is produced from a gene transfected into yeast cells and purified for injection as a subunit vaccine. This is much safer than using attenuated HBV, which could cause lethal hepatitis or liver cancer if it reverted to its virulent phenotype. Recombinant DNA techniques can also be used to make safer attenuated pathogen vaccines

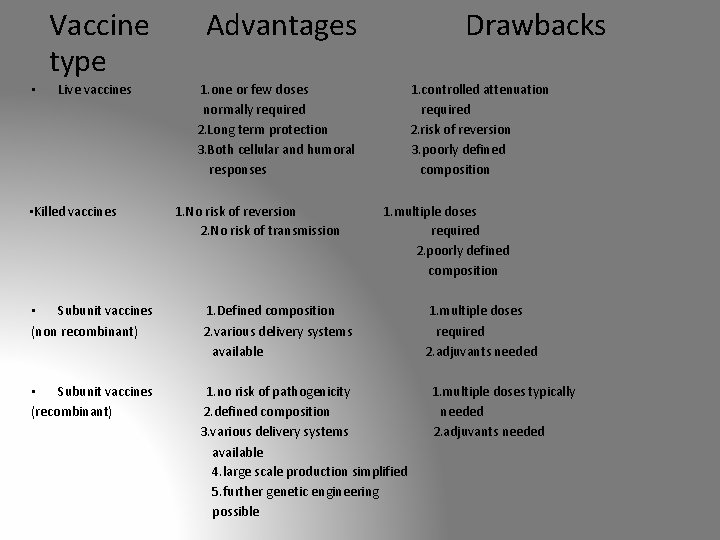
Vaccine type • Live vaccines • Killed vaccines Advantages Drawbacks 1. one or few doses normally required 2. Long term protection 3. Both cellular and humoral responses 1. No risk of reversion 2. No risk of transmission 1. controlled attenuation required 2. risk of reversion 3. poorly defined composition 1. multiple doses required 2. poorly defined composition • Subunit vaccines (non recombinant) 1. Defined composition 2. various delivery systems available • Subunit vaccines (recombinant) 1. no risk of pathogenicity 2. defined composition 3. various delivery systems available 4. large scale production simplified 5. further genetic engineering possible 1. multiple doses required 2. adjuvants needed 1. multiple doses typically needed 2. adjuvants needed

Which Vector to be used? Must be compatible with host cell system (prokaryotic vectors for prokaryotic cells, eukaryotic vectors for eukaryotic cells) Needs a good combination of –strong promoters –ribosome binding sites –termination sequences –affinity tag or solublization sequences –multi-enzyme restriction site

A gene coding for an immunogenic protein from one organism into the genome of other, such as vaccinia virus is introduced. The organism expressing that gene is called as recombinant. Following injection into the subject, the recombinant will replicate and express sufficient amounts of the foreign protein to induce a specific immune response to the protein.

Advantages of viral vector vaccines • Elicit strong humoral and cell-mediated immune responses, resulting in immunological memory. • Can be targeted by viral tropisms for particular cells, e. g. intestine, brain, etc. , inducing desired immunity. • Can also encode for several antigens from different pathogens, introducing the possibility of a single vaccine for several diseases. • Viral vectors have been found not to interfere with the protection produced by other types of vaccines. . • Vaccines are relatively inexpensive and, for some, easily transportable.

Disadvantages • • • Since the live virus being used is an attenuated form of a human pathogen, there is always a risk of reversion to virulence. Some of the vectors under consideration, such as adenovirus, have the capability of transforming cells to a cancerous phenotype. While these oncogenes are removed, vector virus could recombine with naturally occurring, pathogenic strains in the environment and form a new hybrid virus with transforming properties. Immune response to virus-infected cells may cause pathological problems.


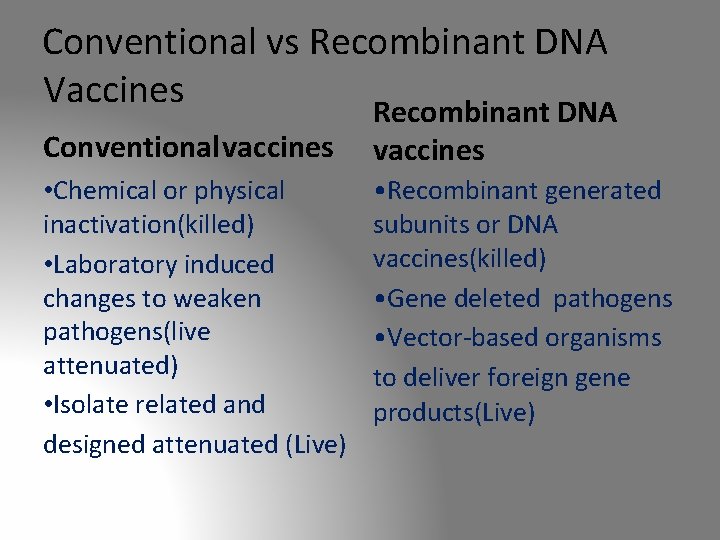
Conventional vs Recombinant DNA Vaccines Conventionalvaccines • Chemical or physical inactivation(killed) • Laboratory induced changes to weaken pathogens(live attenuated) • Isolate related and designed attenuated (Live) Recombinant DNA vaccines • Recombinant generated subunits or DNA vaccines(killed) • Gene deleted pathogens • Vector-based organisms to deliver foreign gene products(Live)

Future Developments • Identification and utilization of better immunogens as new vaccines for diseases • Better vaccine delivery methods: oral, intranasal, and systems allowing mass vaccinations • Use of immunomodulators in vector-based vaccines: CPG motifs and cytokines. • Expression of foreign proteins in plants and the development of edible vaccines • Vaccines developed for non-infectious agents: control and prevent cancer; vaccines to induce long lasting contraception

References • Sciencedirect. com • Academia. com • Animal Agriculture's Future through Biotechnology- Mark W. Jackwood, Leslie Hickle • Essentials of Clinical. Immunology-A. V Hoffbrand
 Cauvery college for women
Cauvery college for women Speed stacks spreads nationally in 1998.
Speed stacks spreads nationally in 1998. Hamlet act iii scene ii
Hamlet act iii scene ii What does naeyc accreditation mean
What does naeyc accreditation mean Ra 10912 cpd act of 2016
Ra 10912 cpd act of 2016 Bosch training academy
Bosch training academy Teta contact details
Teta contact details Cosma accredited schools
Cosma accredited schools Is old dominion university accredited
Is old dominion university accredited What is oshs?
What is oshs? Partner portal ruckus
Partner portal ruckus Aapl accredited schools
Aapl accredited schools Accredited organization
Accredited organization Emirates authority for standardization and metrology logo
Emirates authority for standardization and metrology logo Naacls accredited cytogenetics education program
Naacls accredited cytogenetics education program Uc berkeley extension
Uc berkeley extension Ascld lab
Ascld lab Psgr krishnammal college
Psgr krishnammal college Autonomous expenditure formula
Autonomous expenditure formula Government expenditure multiplier formula
Government expenditure multiplier formula Autonomous benthic explorer
Autonomous benthic explorer What theories
What theories Autonomous and conditional specification
Autonomous and conditional specification