Case Discussion A 52 yearold man initially presented





- Slides: 28

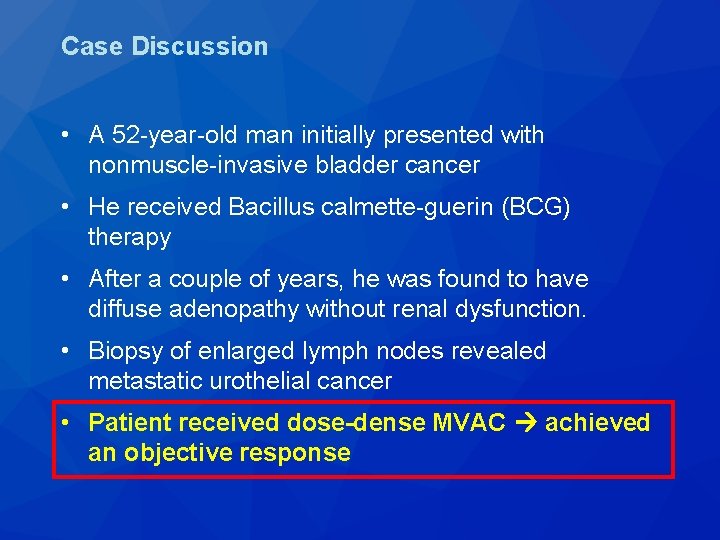
Case Discussion • A 52 -year-old man initially presented with nonmuscle-invasive bladder cancer • He received Bacillus calmette-guerin (BCG) therapy • After a couple of years, he was found to have diffuse adenopathy without renal dysfunction. • Biopsy of enlarged lymph nodes revealed metastatic urothelial cancer

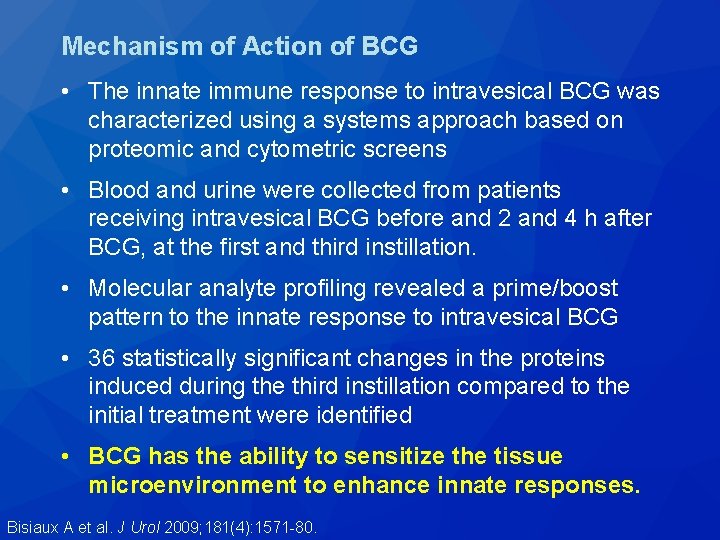
Mechanism of Action of BCG • The innate immune response to intravesical BCG was characterized using a systems approach based on proteomic and cytometric screens • Blood and urine were collected from patients receiving intravesical BCG before and 2 and 4 h after BCG, at the first and third instillation. • Molecular analyte profiling revealed a prime/boost pattern to the innate response to intravesical BCG • 36 statistically significant changes in the proteins induced during the third instillation compared to the initial treatment were identified • BCG has the ability to sensitize the tissue microenvironment to enhance innate responses. Bisiaux A et al. J Urol 2009; 181(4): 1571 -80.

Case Discussion • A 52 -year-old man initially presented with nonmuscle-invasive bladder cancer • He received Bacillus calmette-guerin (BCG) therapy • After a couple of years, he was found to have diffuse adenopathy without renal dysfunction. • Biopsy of enlarged lymph nodes revealed metastatic urothelial cancer • Patient received dose-dense MVAC achieved an objective response

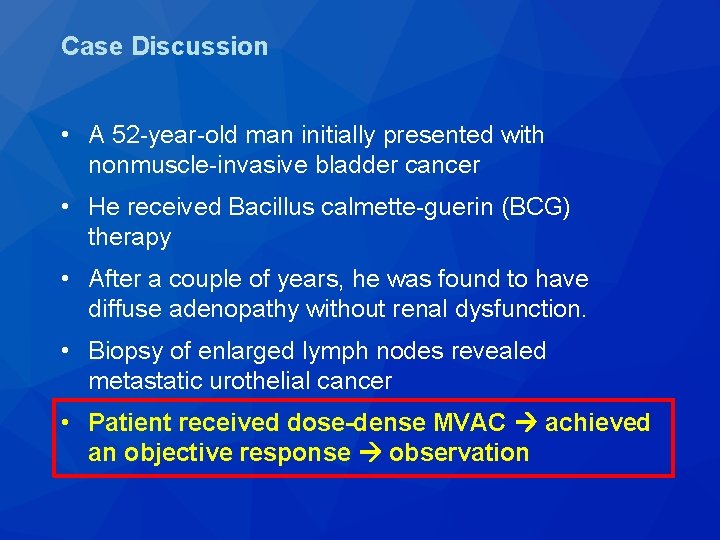
Case Discussion • A 52 -year-old man initially presented with nonmuscle-invasive bladder cancer • He received Bacillus calmette-guerin (BCG) therapy • After a couple of years, he was found to have diffuse adenopathy without renal dysfunction. • Biopsy of enlarged lymph nodes revealed metastatic urothelial cancer • Patient received dose-dense MVAC achieved an objective response observation

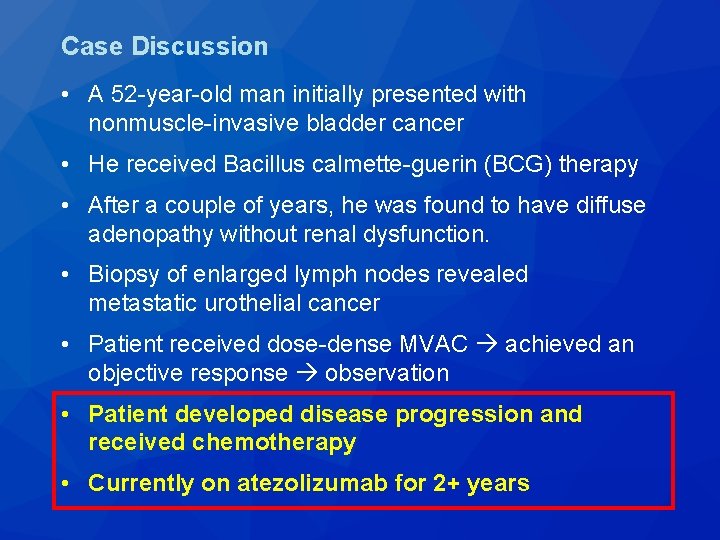
Case Discussion • A 52 -year-old man initially presented with nonmuscle-invasive bladder cancer • He received Bacillus calmette-guerin (BCG) therapy • After a couple of years, he was found to have diffuse adenopathy without renal dysfunction. • Biopsy of enlarged lymph nodes revealed metastatic urothelial cancer • Patient received dose-dense MVAC achieved an objective response observation • Patient developed disease progression and received chemotherapy • Currently on atezolizumab for 2+ years

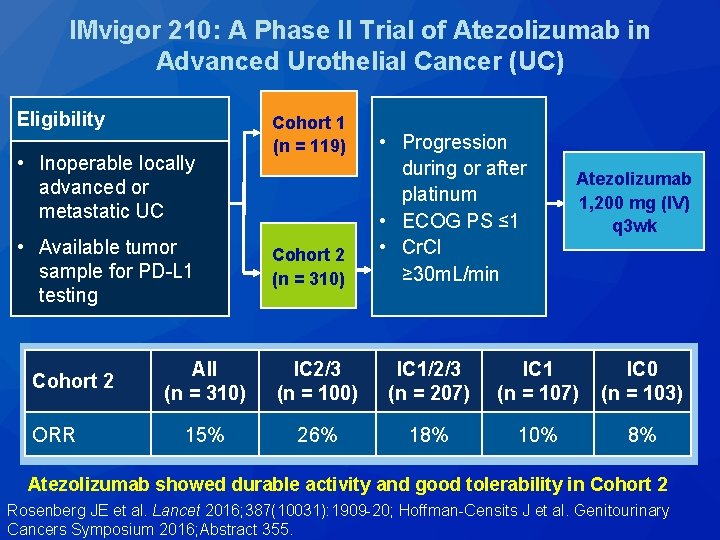
IMvigor 210: A Phase II Trial of Atezolizumab in Advanced Urothelial Cancer (UC) Eligibility • Inoperable locally advanced or metastatic UC • Available tumor sample for PD-L 1 testing Cohort 2 ORR Cohort 1 (n = 119) Cohort 2 (n = 310) • Progression during or after platinum • ECOG PS ≤ 1 • Cr. Cl ≥ 30 m. L/min Atezolizumab 1, 200 mg (IV) q 3 wk All (n = 310) IC 2/3 (n = 100) IC 1/2/3 (n = 207) IC 1 (n = 107) IC 0 (n = 103) 15% 26% 18% 10% 8% Atezolizumab showed durable activity and good tolerability in Cohort 2 Rosenberg JE et al. Lancet 2016; 387(10031): 1909 -20; Hoffman-Censits J et al. Genitourinary Cancers Symposium 2016; Abstract 355.

Case Discussion • A 52 -year-old man initially presented with nonmuscle-invasive bladder cancer • He received Bacillus calmette-guerin (BCG) therapy • After a couple of years, he was found to have diffuse adenopathy without renal dysfunction. • Biopsy of enlarged lymph nodes revealed metastatic urothelial cancer • Patient received dose-dense MVAC achieved an objective response observation • Patient developed disease progression and received chemotherapy • Currently on atezolizumab for 2+ years

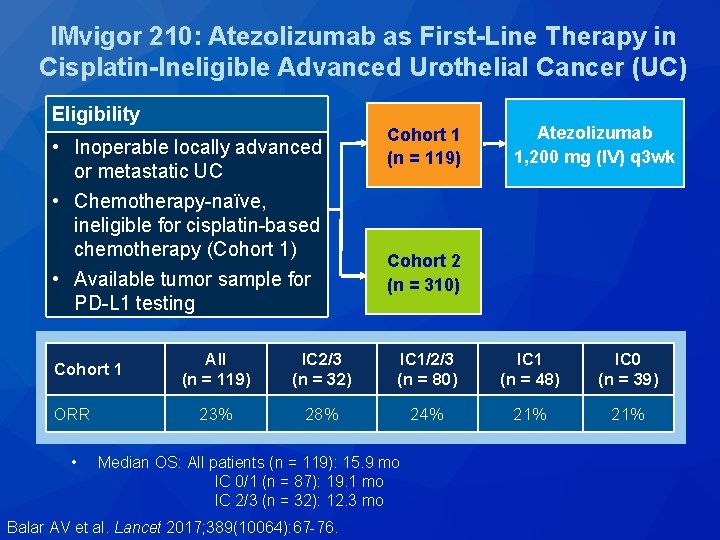
IMvigor 210: Atezolizumab as First-Line Therapy in Cisplatin-Ineligible Advanced Urothelial Cancer (UC) Eligibility • Inoperable locally advanced or metastatic UC • Chemotherapy-naïve, ineligible for cisplatin-based chemotherapy (Cohort 1) • Available tumor sample for PD-L 1 testing Cohort 1 ORR • Cohort 1 (n = 119) Atezolizumab 1, 200 mg (IV) q 3 wk Cohort 2 (n = 310) All (n = 119) IC 2/3 (n = 32) IC 1/2/3 (n = 80) IC 1 (n = 48) IC 0 (n = 39) 23% 28% 24% 21% Median OS: All patients (n = 119): 15. 9 mo IC 0/1 (n = 87): 19. 1 mo IC 2/3 (n = 32): 12. 3 mo Balar AV et al. Lancet 2017; 389(10064): 67 -76.

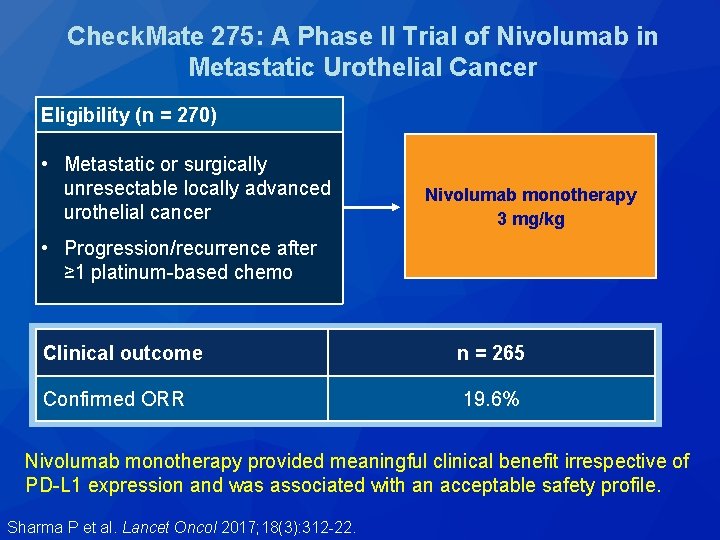
Check. Mate 275: A Phase II Trial of Nivolumab in Metastatic Urothelial Cancer Eligibility (n = 270) • Metastatic or surgically unresectable locally advanced urothelial cancer Nivolumab monotherapy 3 mg/kg • Progression/recurrence after ≥ 1 platinum-based chemo Clinical outcome n = 265 Confirmed ORR 19. 6% Nivolumab monotherapy provided meaningful clinical benefit irrespective of PD-L 1 expression and was associated with an acceptable safety profile. Sharma P et al. Lancet Oncol 2017; 18(3): 312 -22.

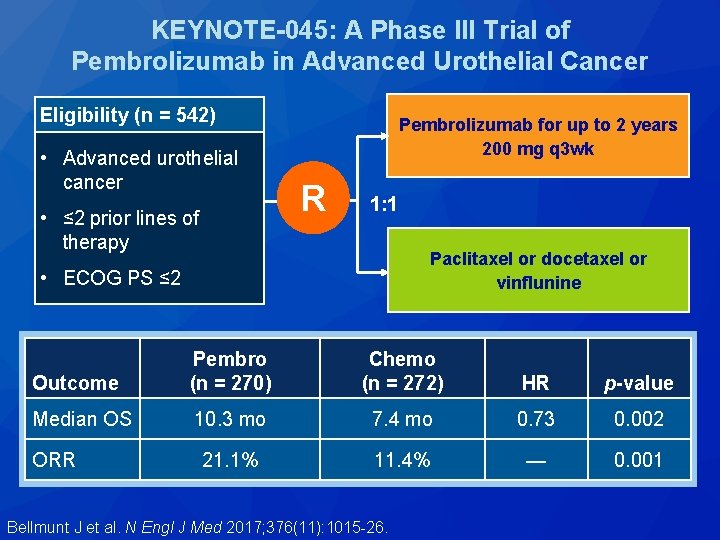
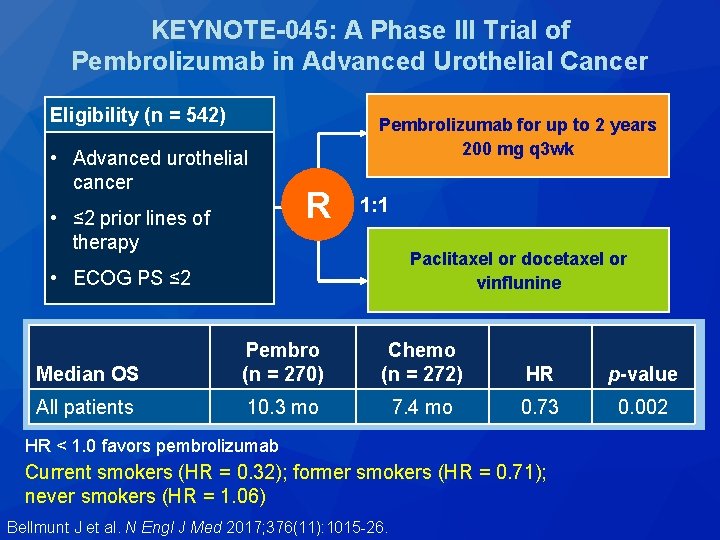
KEYNOTE-045: A Phase III Trial of Pembrolizumab in Advanced Urothelial Cancer Eligibility (n = 542) • Advanced urothelial cancer • ≤ 2 prior lines of therapy Pembrolizumab for up to 2 years 200 mg q 3 wk R 1: 1 Paclitaxel or docetaxel or vinflunine • ECOG PS ≤ 2 Outcome Pembro (n = 270) Chemo (n = 272) HR p-value Median OS 10. 3 mo 7. 4 mo 0. 73 0. 002 21. 1% 11. 4% — 0. 001 ORR Bellmunt J et al. N Engl J Med 2017; 376(11): 1015 -26.

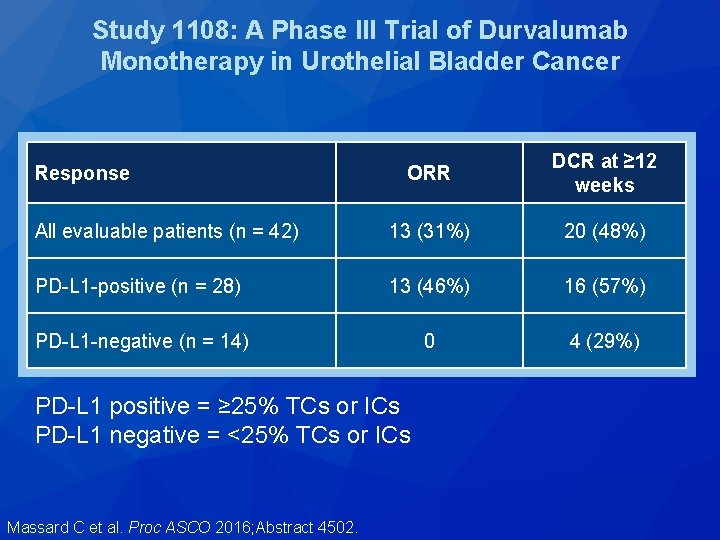
Study 1108: A Phase III Trial of Durvalumab Monotherapy in Urothelial Bladder Cancer ORR DCR at ≥ 12 weeks All evaluable patients (n = 42) 13 (31%) 20 (48%) PD-L 1 -positive (n = 28) 13 (46%) 16 (57%) PD-L 1 -negative (n = 14) 0 4 (29%) Response PD-L 1 positive = ≥ 25% TCs or ICs PD-L 1 negative = <25% TCs or ICs Massard C et al. Proc ASCO 2016; Abstract 4502.

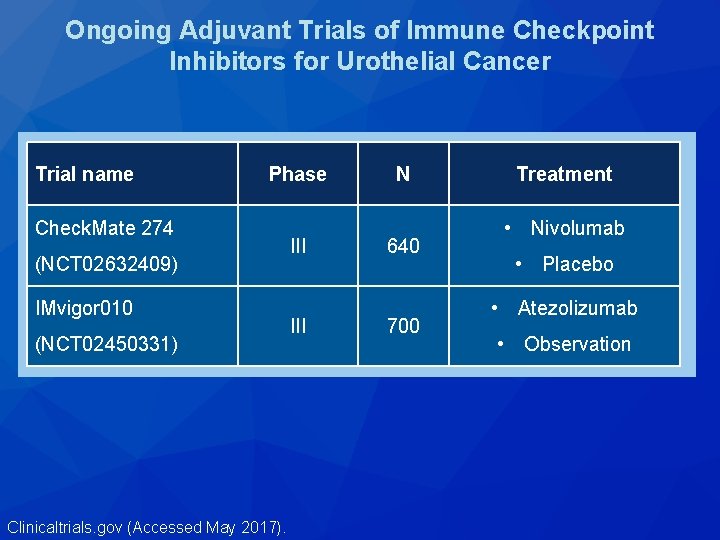
Ongoing Adjuvant Trials of Immune Checkpoint Inhibitors for Urothelial Cancer Trial name Phase Check. Mate 274 (NCT 02632409) IMvigor 010 (NCT 02450331) Clinicaltrials. gov (Accessed May 2017). III N 640 700 Treatment • Nivolumab • Placebo • Atezolizumab • Observation

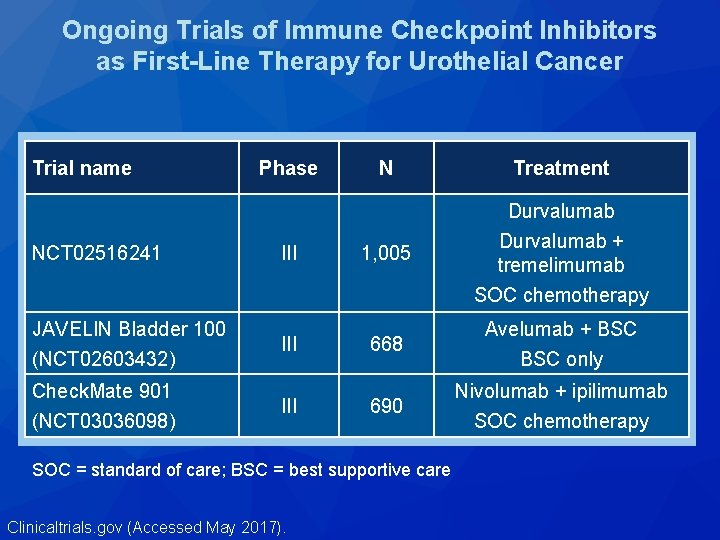
Ongoing Trials of Immune Checkpoint Inhibitors as First-Line Therapy for Urothelial Cancer Trial name NCT 02516241 JAVELIN Bladder 100 (NCT 02603432) Check. Mate 901 (NCT 03036098) Phase III III N Treatment 1, 005 Durvalumab + tremelimumab SOC chemotherapy 668 Avelumab + BSC only 690 Nivolumab + ipilimumab SOC chemotherapy SOC = standard of care; BSC = best supportive care Clinicaltrials. gov (Accessed May 2017).

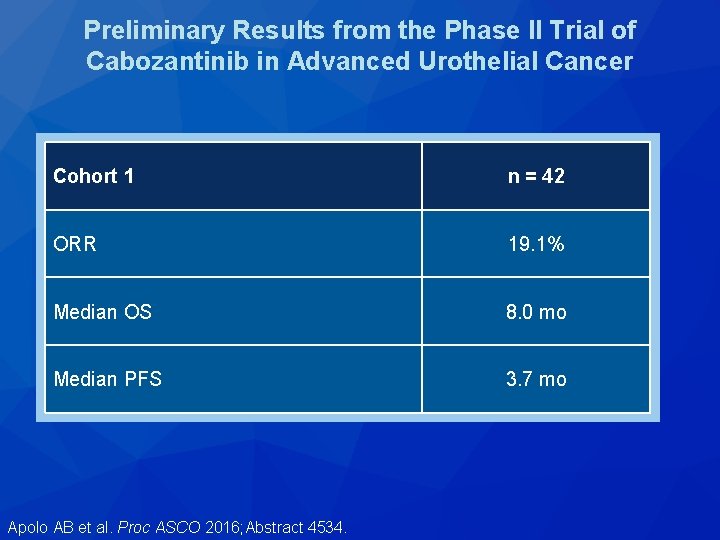
Preliminary Results from the Phase II Trial of Cabozantinib in Advanced Urothelial Cancer Cohort 1 n = 42 ORR 19. 1% Median OS 8. 0 mo Median PFS 3. 7 mo Apolo AB et al. Proc ASCO 2016; Abstract 4534.

KEYNOTE-045: A Phase III Trial of Pembrolizumab in Advanced Urothelial Cancer Eligibility (n = 542) • Advanced urothelial cancer • ≤ 2 prior lines of therapy Pembrolizumab for up to 2 years 200 mg q 3 wk R 1: 1 Paclitaxel or docetaxel or vinflunine • ECOG PS ≤ 2 Median OS Pembro (n = 270) Chemo (n = 272) HR p-value All patients 10. 3 mo 7. 4 mo 0. 73 0. 002 HR < 1. 0 favors pembrolizumab Current smokers (HR = 0. 32); former smokers (HR = 0. 71); never smokers (HR = 1. 06) Bellmunt J et al. N Engl J Med 2017; 376(11): 1015 -26.

Perspective on the Differences Among the Anti-PD-1/PD-L 1 Inhibitors “I don’t think there’s really any difference that we can measure or see based on the data sets we have between any of these inhibitors. I think they all work on the same axis. They all essentially do the same thing. There may be slight differences in toxicity profile, but we’re going to need more data to really sort that out. I think they’re very similar. And I think, also, similar combinations are being looked at, as well, with all of these. ” Elizabeth Plimack, MD

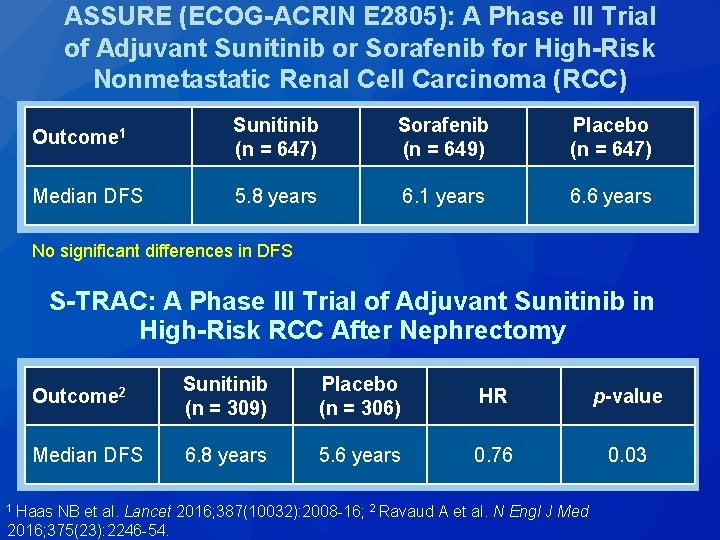
ASSURE (ECOG-ACRIN E 2805): A Phase III Trial of Adjuvant Sunitinib or Sorafenib for High-Risk Nonmetastatic Renal Cell Carcinoma (RCC) Outcome 1 Sunitinib (n = 647) Sorafenib (n = 649) Placebo (n = 647) Median DFS 5. 8 years 6. 1 years 6. 6 years No significant differences in DFS S-TRAC: A Phase III Trial of Adjuvant Sunitinib in High-Risk RCC After Nephrectomy Outcome 2 Sunitinib (n = 309) Placebo (n = 306) HR p-value Median DFS 6. 8 years 5. 6 years 0. 76 0. 03 1 Haas NB et al. Lancet 2016; 375(23): 2246 -54. 2016; 387(10032): 2008 -16; 2 Ravaud A et al. N Engl J Med

Management of Sunitinib-Associated Hand-Foot Skin Reaction (HFSR) HFSR really interferes with people’s activities of daily living. • We are very proactive with having the patients see a dermatologist who’s well versed with the management of HFSR. • We maximize the use of topical ointments. • We encourage the use of soft-soled shoes. • However, if it continues to be problematic, we dose reduce because the patients need to be active and have a good quality of life. Robert J Motzer, MD

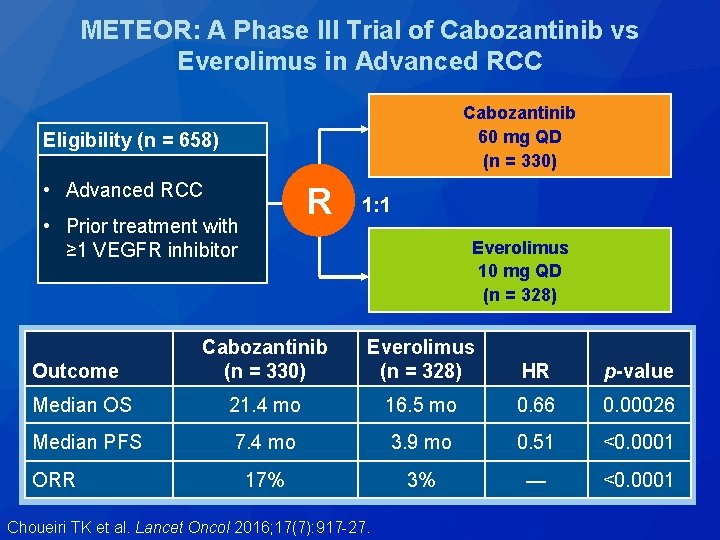
METEOR: A Phase III Trial of Cabozantinib vs Everolimus in Advanced RCC Cabozantinib 60 mg QD (n = 330) Eligibility (n = 658) • Advanced RCC R • Prior treatment with ≥ 1 VEGFR inhibitor 1: 1 Everolimus 10 mg QD (n = 328) Cabozantinib (n = 330) Everolimus (n = 328) HR p-value Median OS 21. 4 mo 16. 5 mo 0. 66 0. 00026 Median PFS 7. 4 mo 3. 9 mo 0. 51 <0. 0001 17% 3% — <0. 0001 Outcome ORR Choueiri TK et al. Lancet Oncol 2016; 17(7): 917 -27.

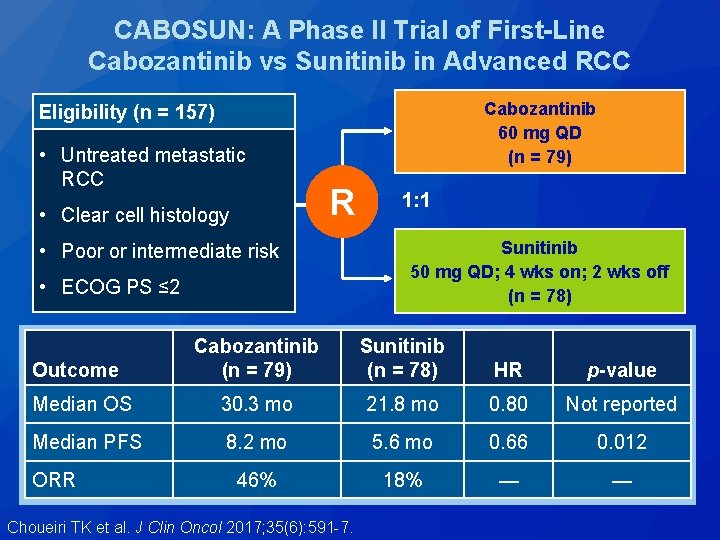
CABOSUN: A Phase II Trial of First-Line Cabozantinib vs Sunitinib in Advanced RCC Cabozantinib 60 mg QD (n = 79) Eligibility (n = 157) • Untreated metastatic RCC • Clear cell histology R • Poor or intermediate risk • ECOG PS ≤ 2 1: 1 Sunitinib 50 mg QD; 4 wks on; 2 wks off (n = 78) Cabozantinib (n = 79) Sunitinib (n = 78) HR p-value Median OS 30. 3 mo 21. 8 mo 0. 80 Not reported Median PFS 8. 2 mo 5. 6 mo 0. 66 0. 012 46% 18% — — Outcome ORR Choueiri TK et al. J Clin Oncol 2017; 35(6): 591 -7.

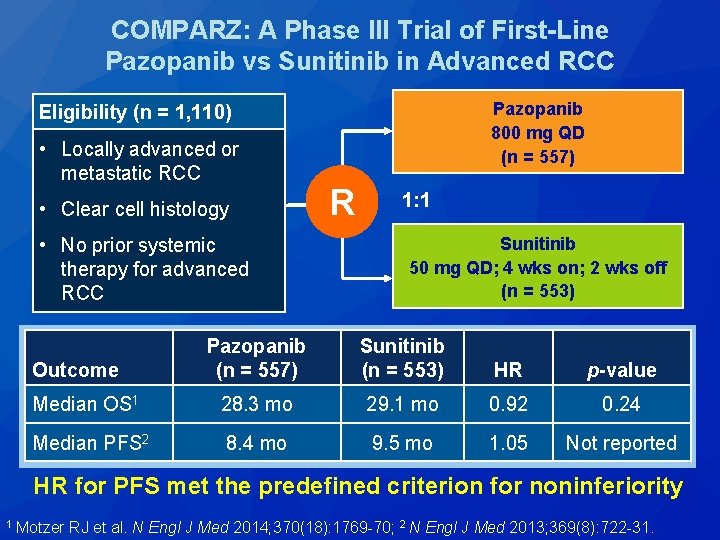
COMPARZ: A Phase III Trial of First-Line Pazopanib vs Sunitinib in Advanced RCC Pazopanib 800 mg QD (n = 557) Eligibility (n = 1, 110) • Locally advanced or metastatic RCC • Clear cell histology • No prior systemic therapy for advanced RCC R 1: 1 Sunitinib 50 mg QD; 4 wks on; 2 wks off (n = 553) Pazopanib (n = 557) Sunitinib (n = 553) HR p-value Median OS 1 28. 3 mo 29. 1 mo 0. 92 0. 24 Median PFS 2 8. 4 mo 9. 5 mo 1. 05 Not reported Outcome HR for PFS met the predefined criterion for noninferiority 1 Motzer RJ et al. N Engl J Med 2014; 370(18): 1769 -70; 2 N Engl J Med 2013; 369(8): 722 -31.

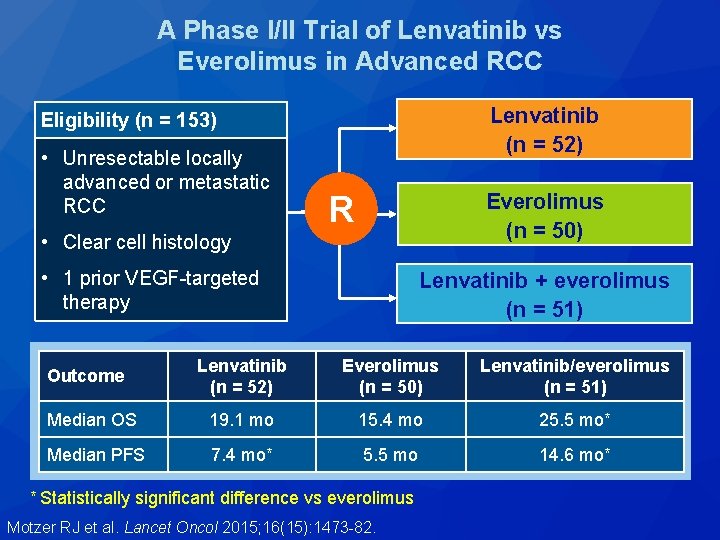
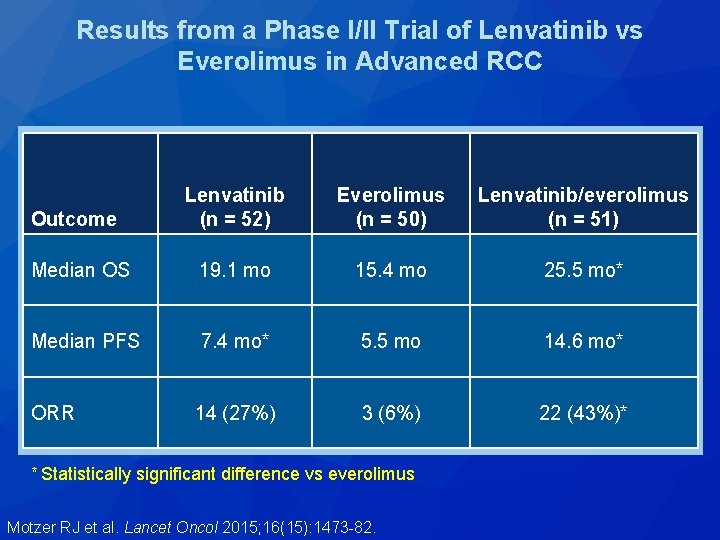
A Phase I/II Trial of Lenvatinib vs Everolimus in Advanced RCC Lenvatinib (n = 52) Eligibility (n = 153) • Unresectable locally advanced or metastatic RCC Everolimus (n = 50) R • Clear cell histology • 1 prior VEGF-targeted therapy Lenvatinib + everolimus (n = 51) Lenvatinib (n = 52) Everolimus (n = 50) Lenvatinib/everolimus (n = 51) Median OS 19. 1 mo 15. 4 mo 25. 5 mo* Median PFS 7. 4 mo* 5. 5 mo 14. 6 mo* Outcome * Statistically significant difference vs everolimus Motzer RJ et al. Lancet Oncol 2015; 16(15): 1473 -82.

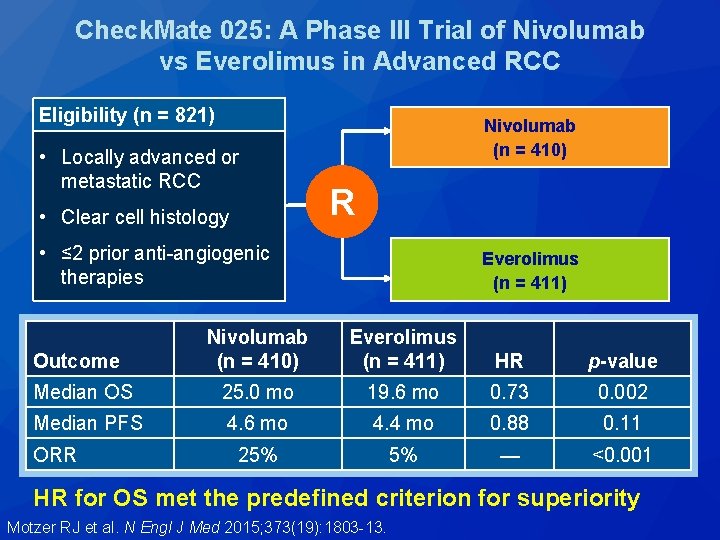
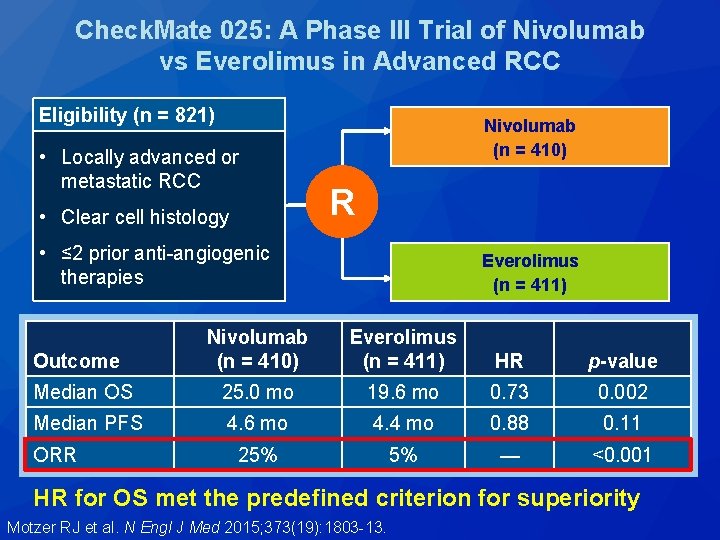
Check. Mate 025: A Phase III Trial of Nivolumab vs Everolimus in Advanced RCC Eligibility (n = 821) • Locally advanced or metastatic RCC • Clear cell histology Nivolumab (n = 410) R • ≤ 2 prior anti-angiogenic therapies Everolimus (n = 411) Nivolumab (n = 410) Everolimus (n = 411) HR p-value Median OS 25. 0 mo 19. 6 mo 0. 73 0. 002 Median PFS 4. 6 mo 4. 4 mo 0. 88 0. 11 25% 5% — <0. 001 Outcome ORR HR for OS met the predefined criterion for superiority Motzer RJ et al. N Engl J Med 2015; 373(19): 1803 -13.

Toxicities Associated with Immune Checkpoint Inhibitors • Colitis • Hypophysitis • Hypothyroidism • Adrenal insufficiency • Dermatologic side effects • Neuropathies • Hepatitis • Pneumonitis

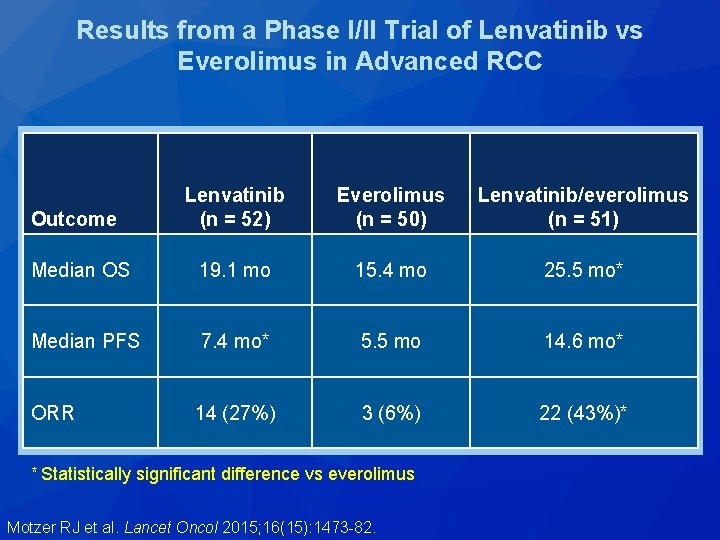
Results from a Phase I/II Trial of Lenvatinib vs Everolimus in Advanced RCC Lenvatinib (n = 52) Everolimus (n = 50) Lenvatinib/everolimus (n = 51) Median OS 19. 1 mo 15. 4 mo 25. 5 mo* Median PFS 7. 4 mo* 5. 5 mo 14. 6 mo* 14 (27%) 3 (6%) 22 (43%)* Outcome ORR * Statistically significant difference vs everolimus Motzer RJ et al. Lancet Oncol 2015; 16(15): 1473 -82.

Results from a Phase I/II Trial of Lenvatinib vs Everolimus in Advanced RCC Lenvatinib (n = 52) Everolimus (n = 50) Lenvatinib/everolimus (n = 51) Median OS 19. 1 mo 15. 4 mo 25. 5 mo* Median PFS 7. 4 mo* 5. 5 mo 14. 6 mo* 14 (27%) 3 (6%) 22 (43%)* Outcome ORR * Statistically significant difference vs everolimus Motzer RJ et al. Lancet Oncol 2015; 16(15): 1473 -82.

Check. Mate 025: A Phase III Trial of Nivolumab vs Everolimus in Advanced RCC Eligibility (n = 821) • Locally advanced or metastatic RCC • Clear cell histology Nivolumab (n = 410) R • ≤ 2 prior anti-angiogenic therapies Everolimus (n = 411) Nivolumab (n = 410) Everolimus (n = 411) HR p-value Median OS 25. 0 mo 19. 6 mo 0. 73 0. 002 Median PFS 4. 6 mo 4. 4 mo 0. 88 0. 11 25% 5% — <0. 001 Outcome ORR HR for OS met the predefined criterion for superiority Motzer RJ et al. N Engl J Med 2015; 373(19): 1803 -13.

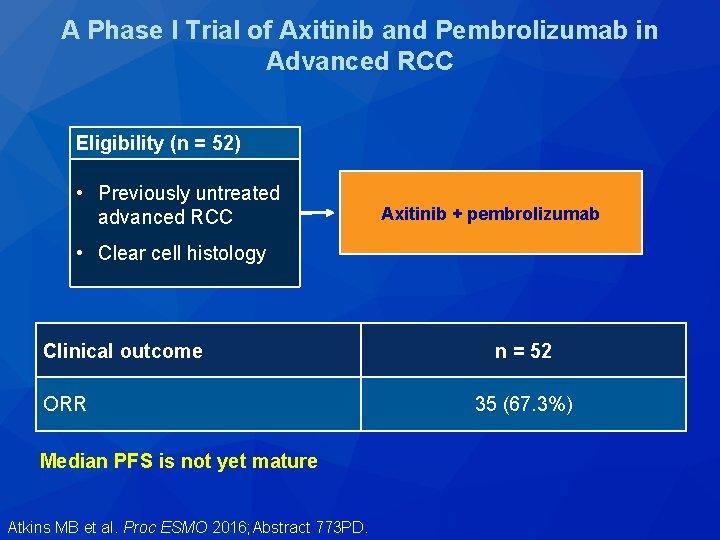
A Phase I Trial of Axitinib and Pembrolizumab in Advanced RCC Eligibility (n = 52) • Previously untreated advanced RCC Axitinib + pembrolizumab • Clear cell histology Clinical outcome ORR Median PFS is not yet mature Atkins MB et al. Proc ESMO 2016; Abstract 773 PD. n = 52 35 (67. 3%)