CALORIMETRY Calorimetry the science associated with determining the



- Slides: 8

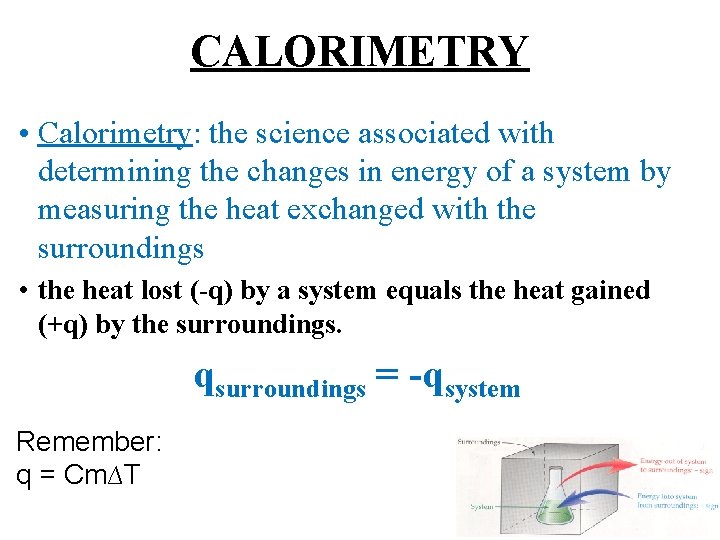
CALORIMETRY • Calorimetry: the science associated with determining the changes in energy of a system by measuring the heat exchanged with the surroundings • the heat lost (-q) by a system equals the heat gained (+q) by the surroundings. qsurroundings = -qsystem Remember: q = Cm∆T

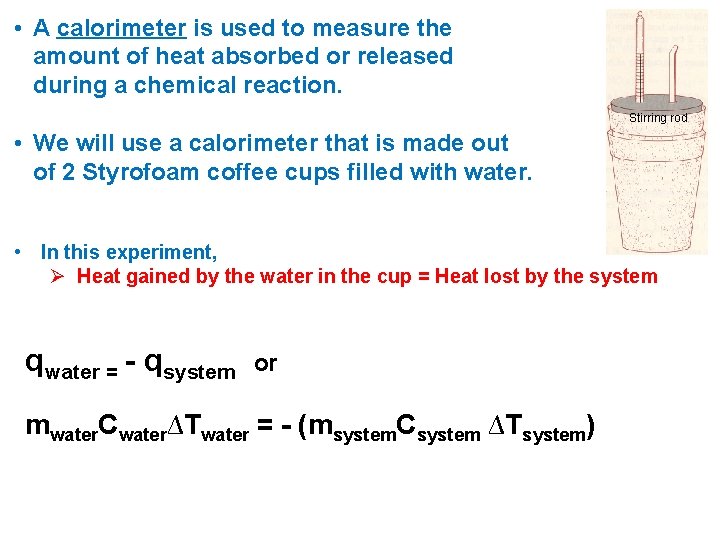
• A calorimeter is used to measure the amount of heat absorbed or released during a chemical reaction. Stirring rod • We will use a calorimeter that is made out of 2 Styrofoam coffee cups filled with water. • In this experiment, Ø Heat gained by the water in the cup = Heat lost by the system qwater = - qsystem or mwater. Cwater∆Twater = - (msystem. Csystem ∆Tsystem)

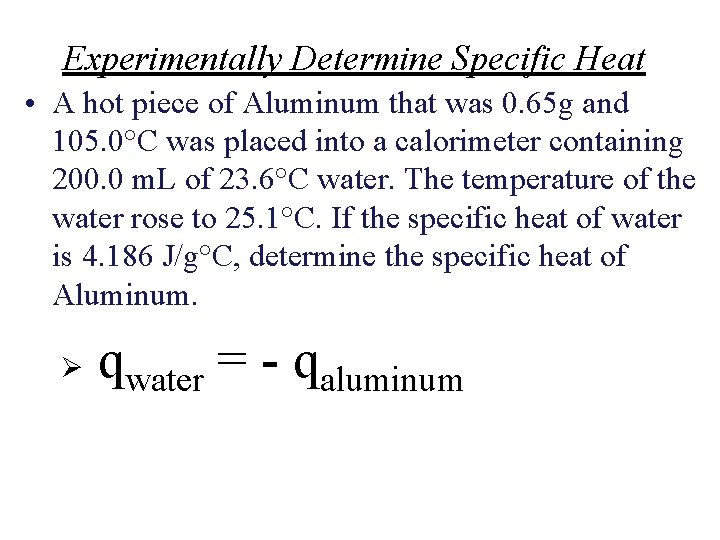
Experimentally Determine Specific Heat • A hot piece of Aluminum that was 0. 65 g and 105. 0°C was placed into a calorimeter containing 200. 0 m. L of 23. 6°C water. The temperature of the water rose to 25. 1°C. If the specific heat of water is 4. 186 J/g°C, determine the specific heat of Aluminum. Ø qwater = - qaluminum

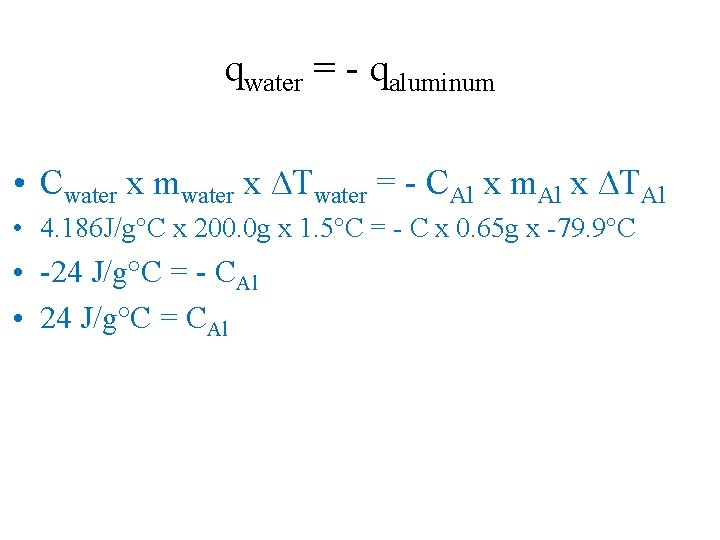
qwater = - qaluminum • Water Ø C = 4. 186 J/g°C Ø Mass = 200. 0 g (200. 0 m. L = 200. 0 g) Ø ∆T = (25. 1°C - 23. 6°C) = 1. 5°C • Alumunium ØC=? ØMass = 0. 65 g Ø∆T = (25. 1°C -105. 0°C) = -79. 9°C

qwater = - qaluminum • Cwater x mwater x ∆Twater = - CAl x m. Al x ∆TAl • 4. 186 J/g°C x 200. 0 g x 1. 5°C = - C x 0. 65 g x -79. 9°C • -24 J/g°C = - CAl • 24 J/g°C = CAl

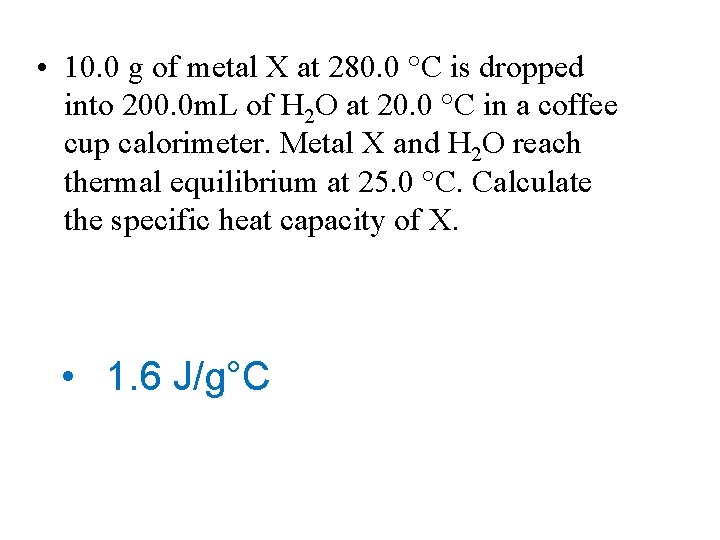
• 10. 0 g of metal X at 280. 0 °C is dropped into 200. 0 m. L of H 2 O at 20. 0 °C in a coffee cup calorimeter. Metal X and H 2 O reach thermal equilibrium at 25. 0 °C. Calculate the specific heat capacity of X. • 1. 6 J/g°C

• A 30. 0 g hot piece of lead was dropped into a calorimeter containing 50. 0 m. L of water at 19. 5°C. After reaching equilibrium, the temperature of the water was 27. 8°C. – How much energy did the water absorb? – What was the original temperature of the lead? • (C lead = 0. 128 J/g°C)

• You put a 25. 0 g piece of zinc in a beaker containing 520. 0 m. L of boiling water. You then take the zinc and put it into a calorimeter containing 150. 0 m. L of water at 21. 7°C. The temperature of the water in the calorimeter rises to 24. 3°C. What is the specific heat of zinc?