An addition to the disaster victim identification investigators







- Slides: 24

An addition to the disaster victim identification investigators toolkit? by E. A. M. Graham, E. E. Turk and G. N. Rutty Forensic Science International : Genetics vol. 2 (2008) pp. 29 -34

Mass fatality incidents

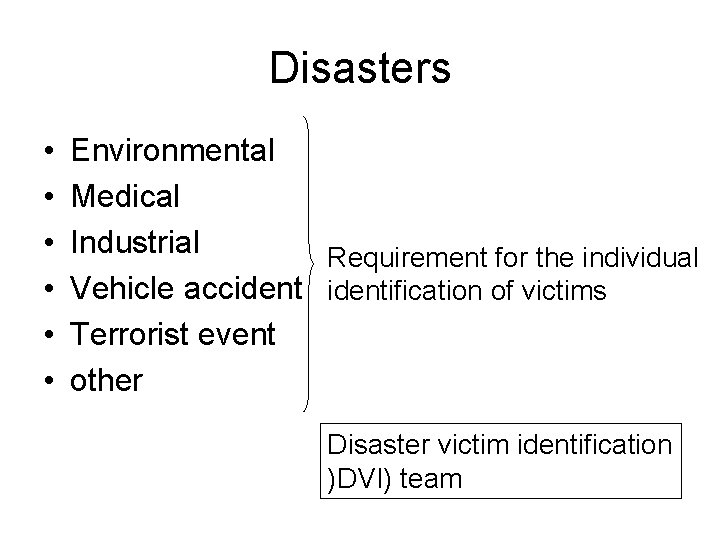
Disasters • • • Environmental Medical Industrial Requirement for the individual Vehicle accidentification of victims Terrorist event other Disaster victim identification )DVI) team

Example environmental event Indian Ocean Tsunami : on 26 th December 2004 -Death of over 200, 000 -Little or no fragmention but decomposition and putrefaction -Occurred due to the tropical climate

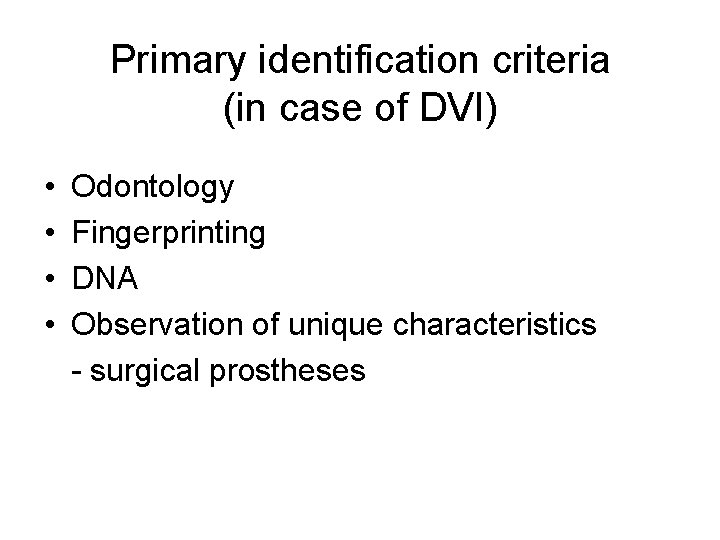
Primary identification criteria (in case of DVI) • • Odontology Fingerprinting DNA Observation of unique characteristics - surgical prostheses

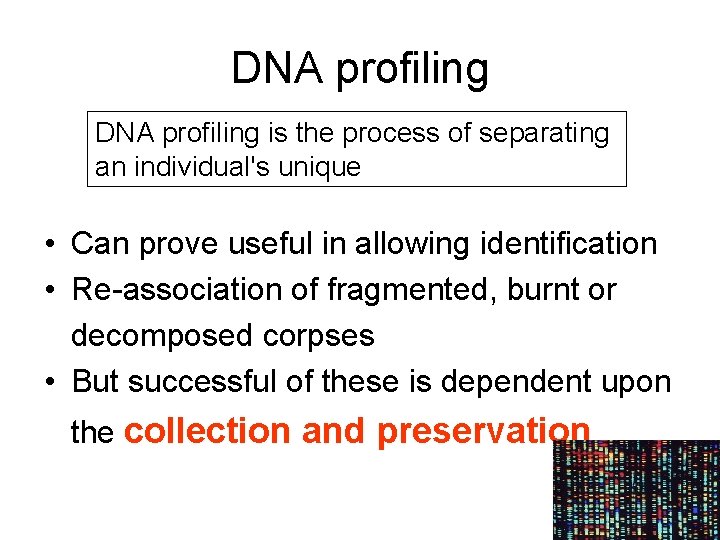
DNA profiling is the process of separating an individual's unique • Can prove useful in allowing identification • Re-association of fragmented, burnt or decomposed corpses • But successful of these is dependent upon the collection and preservation

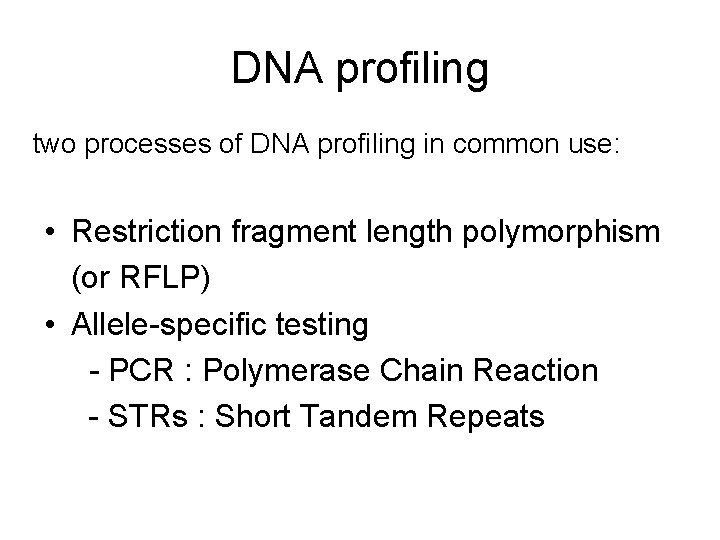
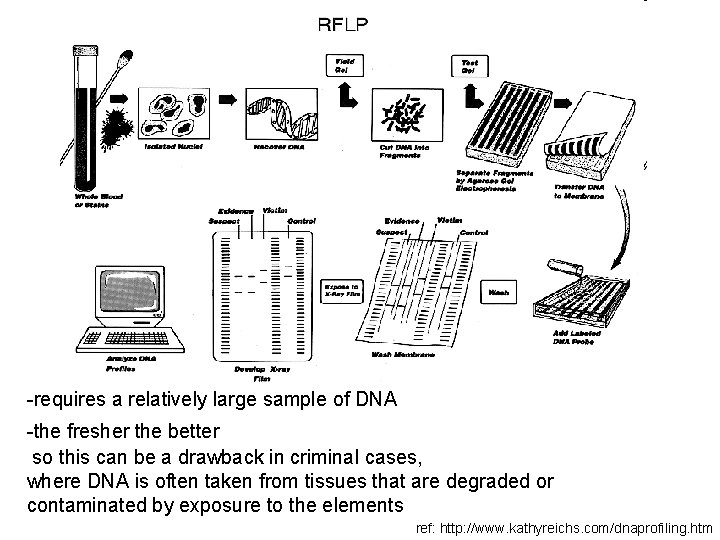
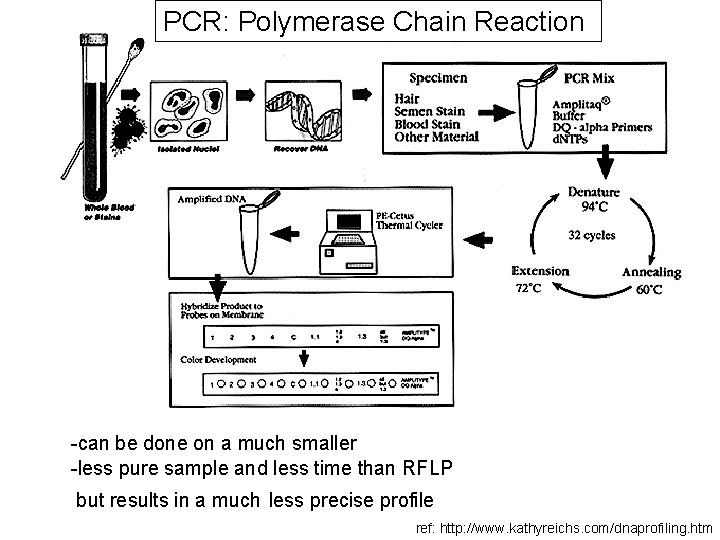
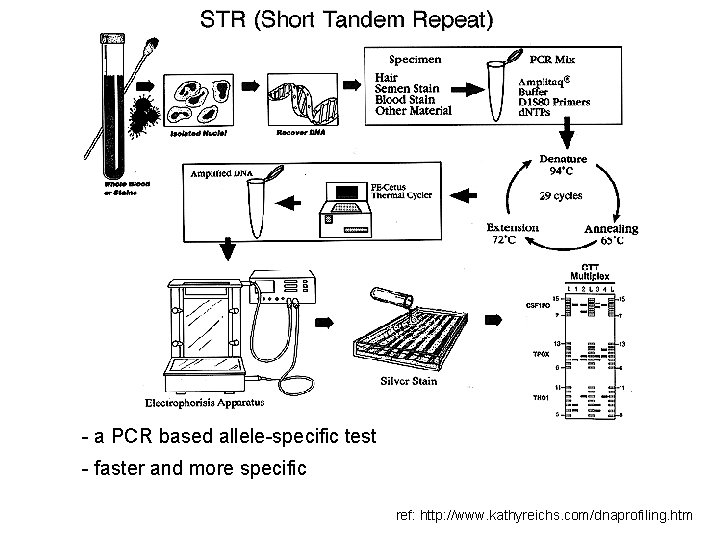
DNA profiling two processes of DNA profiling in common use: • Restriction fragment length polymorphism (or RFLP) • Allele-specific testing - PCR : Polymerase Chain Reaction - STRs : Short Tandem Repeats

-requires a relatively large sample of DNA -the fresher the better so this can be a drawback in criminal cases, where DNA is often taken from tissues that are degraded or contaminated by exposure to the elements ref: http: //www. kathyreichs. com/dnaprofiling. htm

PCR: Polymerase Chain Reaction -can be done on a much smaller -less pure sample and less time than RFLP but results in a much less precise profile ref: http: //www. kathyreichs. com/dnaprofiling. htm

- a PCR based allele-specific test - faster and more specific ref: http: //www. kathyreichs. com/dnaprofiling. htm

Why preserve samples at room temperature? • Usually, samples stored at -20 oc to halt the degradation processes but additional processing of samples is required • Sometime refrigeration is not immediately available • Transportation of samples from one country to another may required

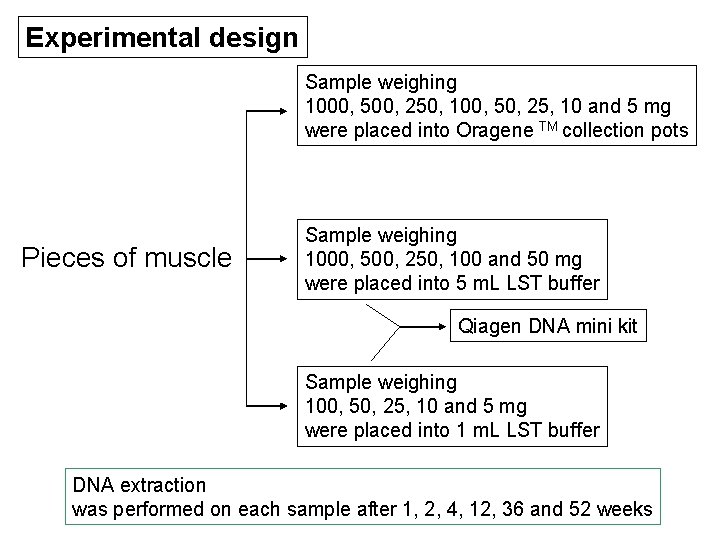
Experiment • Sample collection - obtained from patients donation of their two lower limbs (were amputated due to chronic lower leg ischemia caused by diabetes) [samples is muscle (soft tissue type)] • Preservation methods - lysis storage - transportation (LST) buffer - Oragene TM DNA self-collection kit • Experimental design

Experimental design Sample weighing 1000, 500, 250, 100, 50, 25, 10 and 5 mg were placed into Oragene TM collection pots Pieces of muscle Sample weighing 1000, 500, 250, 100 and 50 mg were placed into 5 m. L LST buffer Qiagen DNA mini kit Sample weighing 100, 50, 25, 10 and 5 mg were placed into 1 m. L LST buffer DNA extraction was performed on each sample after 1, 2, 4, 12, 36 and 52 weeks

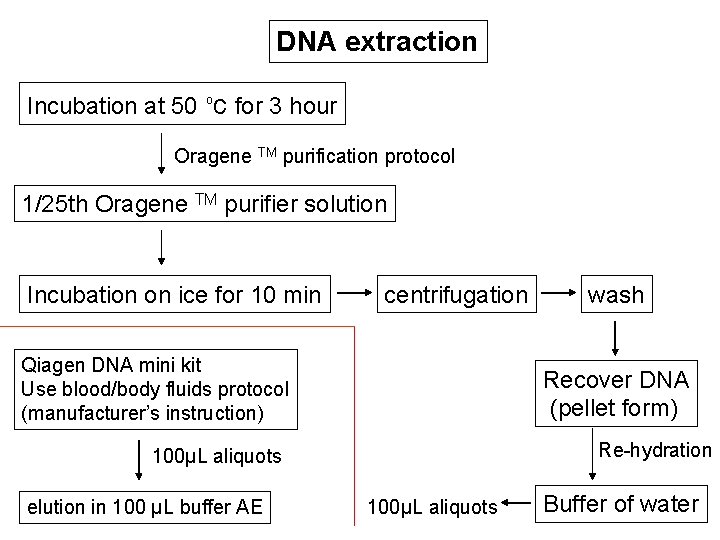
DNA extraction Incubation at 50 oc for 3 hour Oragene TM purification protocol 1/25 th Oragene TM purifier solution Incubation on ice for 10 min centrifugation Qiagen DNA mini kit Use blood/body fluids protocol (manufacturer’s instruction) Recover DNA (pellet form) Re-hydration 100µL aliquots elution in 100 µL buffer AE wash 100µL aliquots Buffer of water

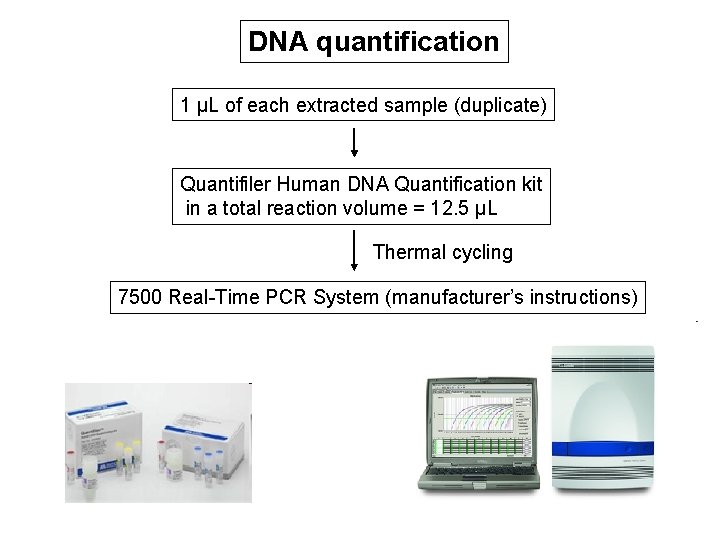
DNA quantification 1 µL of each extracted sample (duplicate) Quantifiler Human DNA Quantification kit in a total reaction volume = 12. 5 µL Thermal cycling 7500 Real-Time PCR System (manufacturer’s instructions)

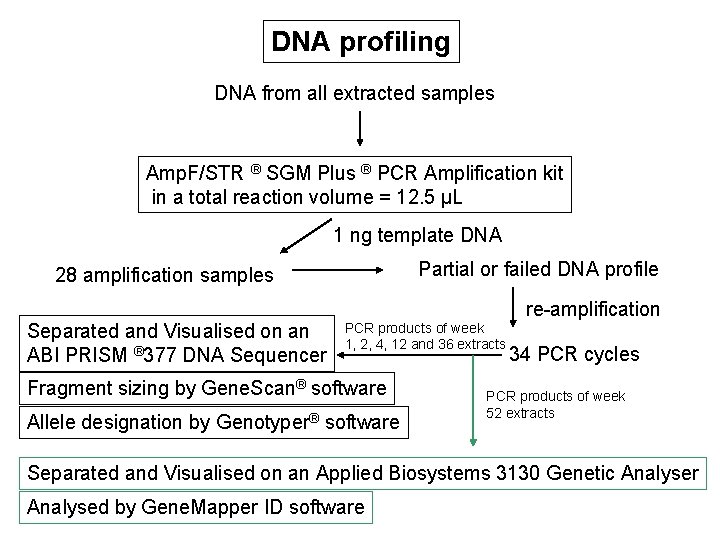
DNA profiling DNA from all extracted samples Amp. F/STR ® SGM Plus ® PCR Amplification kit in a total reaction volume = 12. 5 µL 1 ng template DNA Partial or failed DNA profile 28 amplification samples re-amplification Separated and Visualised on an ABI PRISM ® 377 DNA Sequencer PCR products of week 1, 2, 4, 12 and 36 extracts Fragment sizing by Gene. Scan® software Allele designation by Genotyper® software 34 PCR cycles PCR products of week 52 extracts Separated and Visualised on an Applied Biosystems 3130 Genetic Analyser Analysed by Gene. Mapper ID software

Results

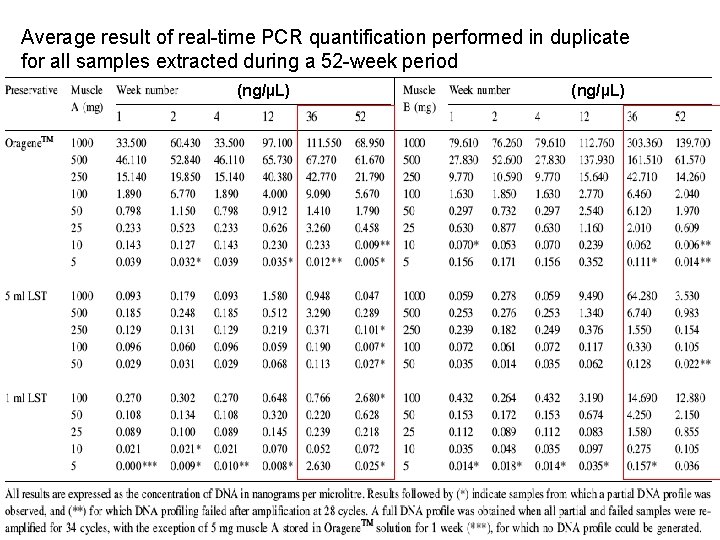
Average result of real-time PCR quantification performed in duplicate for all samples extracted during a 52 -week period (ng/µL)

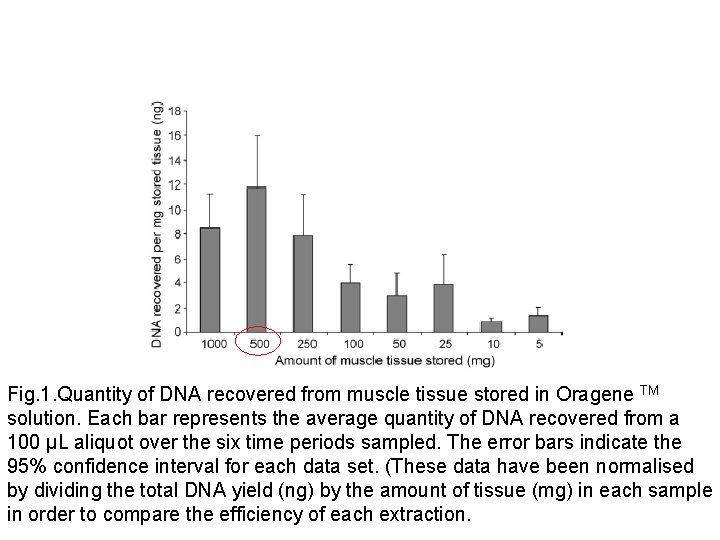
Fig. 1. Quantity of DNA recovered from muscle tissue stored in Oragene TM solution. Each bar represents the average quantity of DNA recovered from a 100 µL aliquot over the six time periods sampled. The error bars indicate the 95% confidence interval for each data set. (These data have been normalised by dividing the total DNA yield (ng) by the amount of tissue (mg) in each sample in order to compare the efficiency of each extraction.

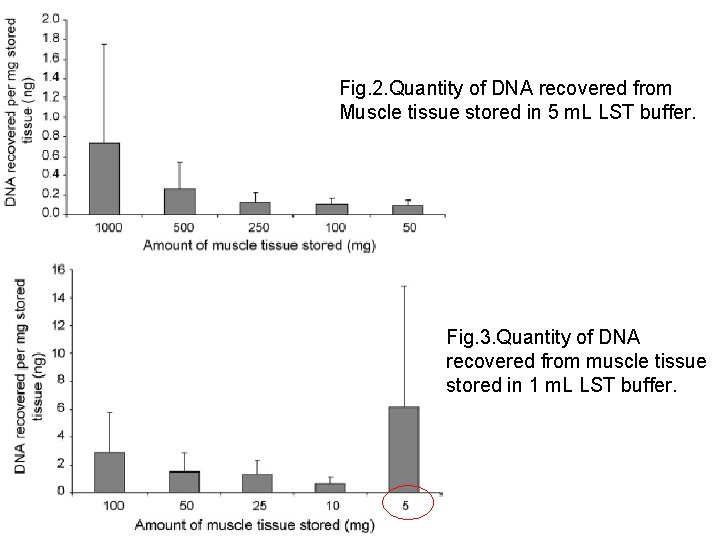
Fig. 2. Quantity of DNA recovered from Muscle tissue stored in 5 m. L LST buffer. Fig. 3. Quantity of DNA recovered from muscle tissue stored in 1 m. L LST buffer.

Conclusion • Quality of DNA recovered from muscle tissue stored in both Oragene TM and LST preservative buffers may begin to diminish after 6 months at room temperature. • Quality of DNA recovered from tissue stored in LST buffer is not significantly reduced compared with that stored in Oragene TM collection pots. • The yield of DNA per mg of tissue stored in 1 m. L was greater than to 5 m. L LST buffer. • LST buffer is better suited to the preservation of small amounts (<100 mg) of tissue. • Both buffer solutions have shown sufficient DNA preservation over a 12 month period of storage at room temperature.

Conclusion (cont(. • Oragene TM collection pots is superior to LST buffer in recovery of high DNA yield, especially when stored in 5 m. L LST buffer. • DNA preservation in hard tissue (bone or teeth) is superior to soft tissues (muscle) especially when putrefaction but processing of hard tissue is extremely time-consuming and labour-intensive. • Low-cost LST buffer and storage without refrigeration. (no additional processing of tissue is required) • This system could also allow for automation of DNA extraction process by use of robotic platforms such as the Qiagen Biorobot, if required. (increase in sample throughput) • This system allows for the collection of small pieces of muscle (or other soft tissue).

Conclusion (cont(. • This system is fully portable and is compatible with barcoding management systems. • From results of both this work and previously work using similar preservation buffers shows that it is applicable to burnt remains and changes of decomposition. • Promoted for DVI field work and is especially applicable in an incident involving disrupted body parts where traditional DNA samples or teeth and bone may not be readily available for identification and fragment reassociation.

 Disaster victim identification
Disaster victim identification Secure the scene
Secure the scene Fda disqualified list
Fda disqualified list Gafsed
Gafsed Arizona homicide investigators association
Arizona homicide investigators association Blocked random assignment
Blocked random assignment How do investigators package dangerous sharp items
How do investigators package dangerous sharp items Central pocket whorl vs plain whorl
Central pocket whorl vs plain whorl North carolina victim assistance network
North carolina victim assistance network Victim
Victim The wanton victim
The wanton victim Stephen schafer victimology
Stephen schafer victimology Victim of spanish duplicity
Victim of spanish duplicity Criminal thinking errors and their corrections
Criminal thinking errors and their corrections Sycamore programme
Sycamore programme Three-person carry or stretcher lift
Three-person carry or stretcher lift Chest compression for infant 2 rescuer
Chest compression for infant 2 rescuer Secondary survey - first aid
Secondary survey - first aid It is an immediate and temporary care given
It is an immediate and temporary care given State my path
State my path Victim and creator mindset
Victim and creator mindset Nature of victimization
Nature of victimization Preposition of victim
Preposition of victim Are you a fashion victim
Are you a fashion victim Victimology
Victimology