According to Le Chteliers principle when a chemical







- Slides: 33

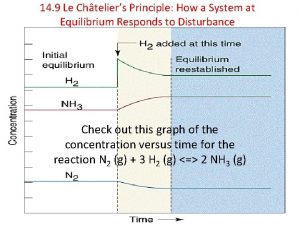
According to Le Châtelier’s principle, when a chemical system at equilibrium is disturbed by a change in property of the system, the system always appears to react to oppose the change, until a new equilibrium is reached. We will examine the effects of changing the • concentrations of reactants or products Henri Louis Le Châtelier (1850 – 1936) • temperature of the system • gas volume (or pressure) 1: 23

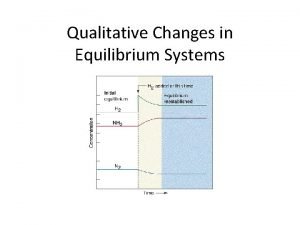
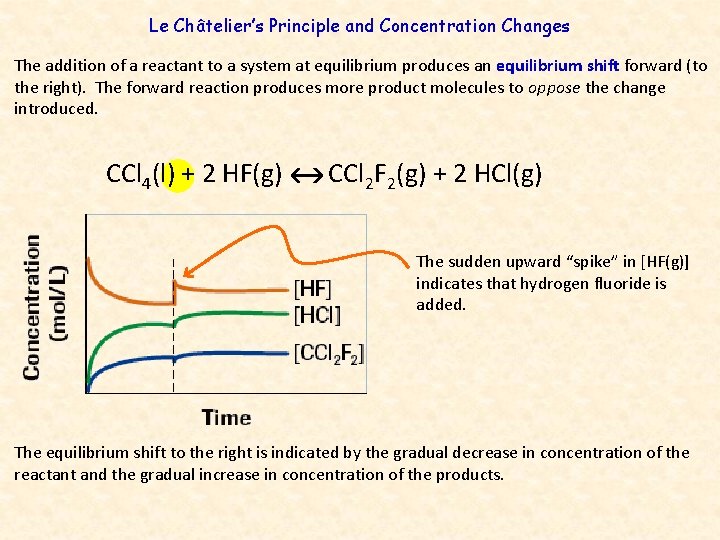
Le Châtelier’s Principle and Concentration Changes The addition of a reactant to a system at equilibrium produces an equilibrium shift forward (to the right). The forward reaction produces more product molecules to oppose the change introduced. CCl 4(l) + 2 HF(g) CCl 2 F 2(g) + 2 HCl(g) The sudden upward “spike” in [HF(g)] indicates that hydrogen fluoride is added. The equilibrium shift to the right is indicated by the gradual decrease in concentration of the reactant and the gradual increase in concentration of the products.

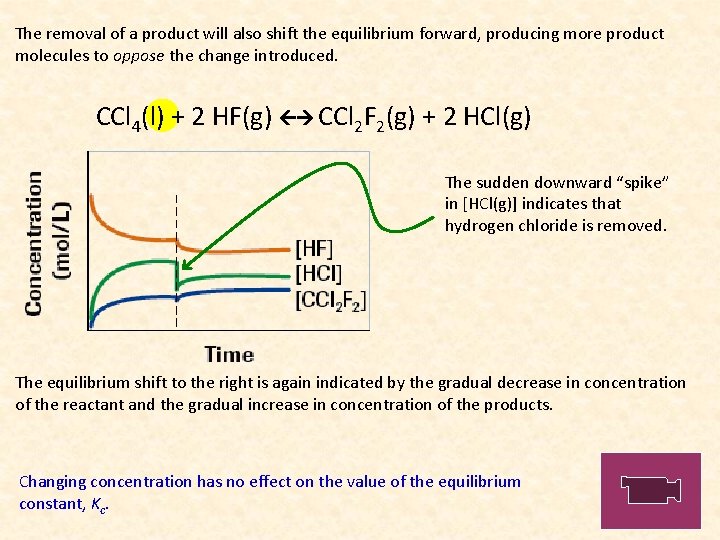
The removal of a product will also shift the equilibrium forward, producing more product molecules to oppose the change introduced. CCl 4(l) + 2 HF(g) CCl 2 F 2(g) + 2 HCl(g) The sudden downward “spike” in [HCl(g)] indicates that hydrogen chloride is removed. The equilibrium shift to the right is again indicated by the gradual decrease in concentration of the reactant and the gradual increase in concentration of the products. Changing concentration has no effect on the value of the equilibrium constant, Kc.

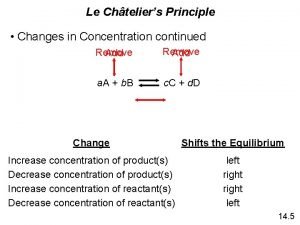
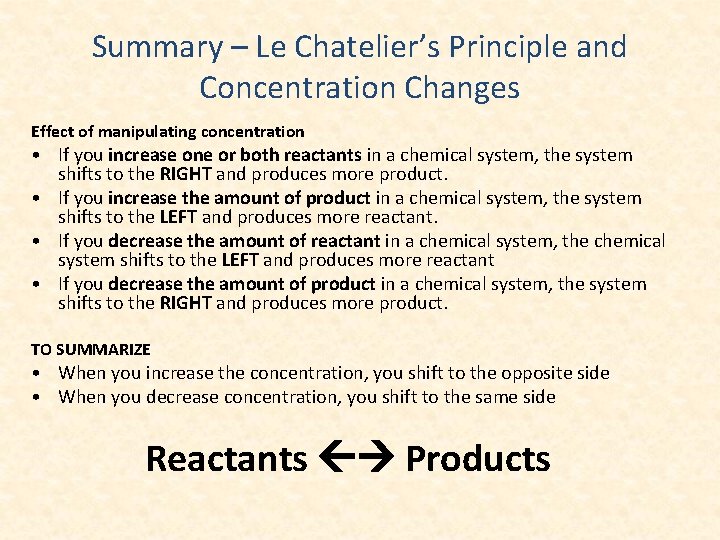
Summary – Le Chatelier’s Principle and Concentration Changes Effect of manipulating concentration • If you increase one or both reactants in a chemical system, the system shifts to the RIGHT and produces more product. • If you increase the amount of product in a chemical system, the system shifts to the LEFT and produces more reactant. • If you decrease the amount of reactant in a chemical system, the chemical system shifts to the LEFT and produces more reactant • If you decrease the amount of product in a chemical system, the system shifts to the RIGHT and produces more product. TO SUMMARIZE • When you increase the concentration, you shift to the opposite side • When you decrease concentration, you shift to the same side Reactants Products

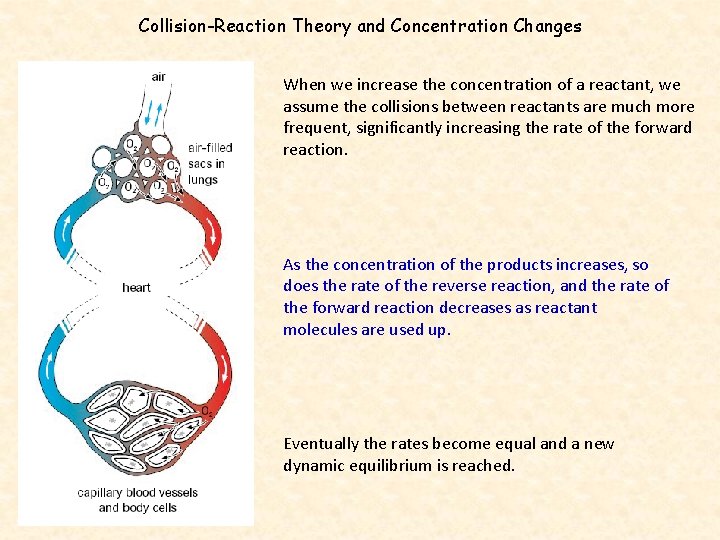
Collision-Reaction Theory and Concentration Changes When we increase the concentration of a reactant, we assume the collisions between reactants are much more frequent, significantly increasing the rate of the forward reaction. As the concentration of the products increases, so does the rate of the reverse reaction, and the rate of the forward reaction decreases as reactant molecules are used up. Eventually the rates become equal and a new dynamic equilibrium is reached.

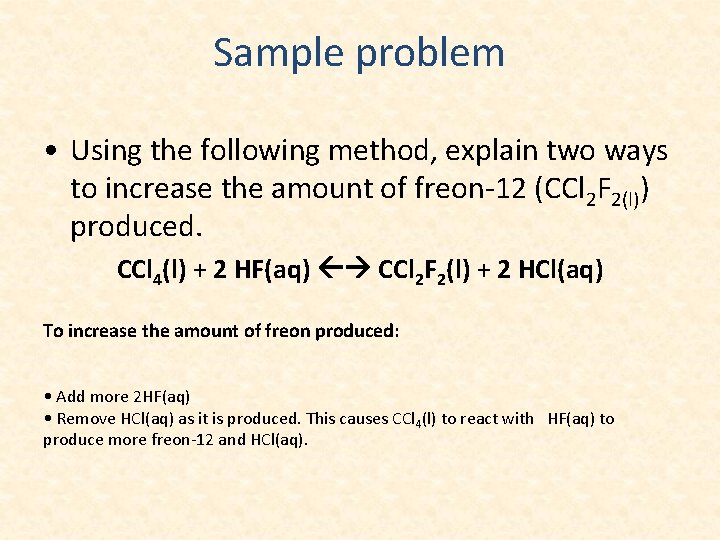
Sample problem • Using the following method, explain two ways to increase the amount of freon-12 (CCl 2 F 2(l)) produced. CCl 4(l) + 2 HF(aq) CCl 2 F 2(l) + 2 HCl(aq) To increase the amount of freon produced: • Add more 2 HF(aq) • Remove HCl(aq) as it is produced. This causes CCl 4(l) to react with HF(aq) to produce more freon-12 and HCl(aq).

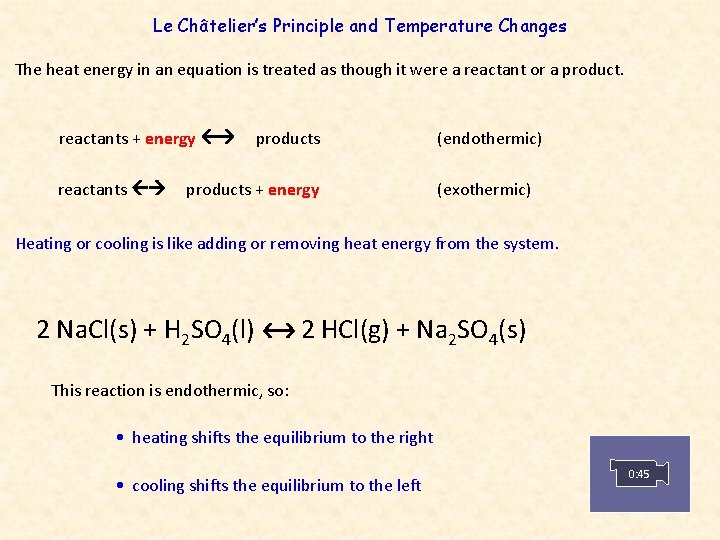
Le Châtelier’s Principle and Temperature Changes The heat energy in an equation is treated as though it were a reactant or a product. reactants + energy reactants products + energy (endothermic) (exothermic) Heating or cooling is like adding or removing heat energy from the system. 2 Na. Cl(s) + H 2 SO 4(l) 2 HCl(g) + Na 2 SO 4(s) This reaction is endothermic, so: • heating shifts the equilibrium to the right • cooling shifts the equilibrium to the left 0: 45

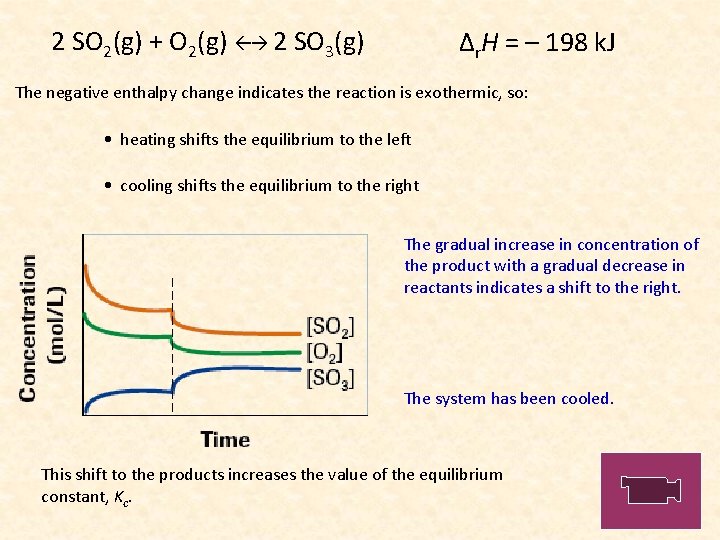
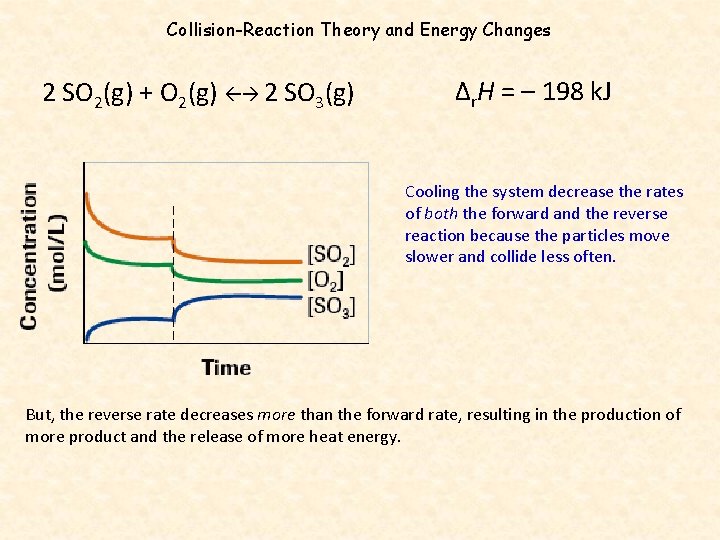
2 SO 2(g) + O 2(g) 2 SO 3(g) Δr. H = – 198 k. J The negative enthalpy change indicates the reaction is exothermic, so: • heating shifts the equilibrium to the left • cooling shifts the equilibrium to the right The gradual increase in concentration of the product with a gradual decrease in reactants indicates a shift to the right. The system has been cooled. This shift to the products increases the value of the equilibrium constant, Kc.

Summary – Le Chatelier’s Principle and Temperature Changes • Once energy is placed on the appropriate side the equation, use the same rules as with manipulating concentration. TO SUMMERIZE • When you increase the amount of energy, shift to the opposite side of the increase • When you decrease the amount of energy, shift to the same side as the decrease

Collision-Reaction Theory and Energy Changes 2 SO 2(g) + O 2(g) 2 SO 3(g) Δr. H = – 198 k. J Cooling the system decrease the rates of both the forward and the reverse reaction because the particles move slower and collide less often. But, the reverse rate decreases more than the forward rate, resulting in the production of more product and the release of more heat energy.

Collision-Reaction Theory and Energy Changes 2 SO 2(g) + O 2(g) 2 SO 3(g) Δr. H = – 198 k. J As energy is being added to a system, particles are moving quicker and as a result, collisions are occurring more frequently increasing both the forward and reverse reaction rates. But, the reverse rate increases more than the forward rate, resulting in the production of more reactants and the release of less heat energy

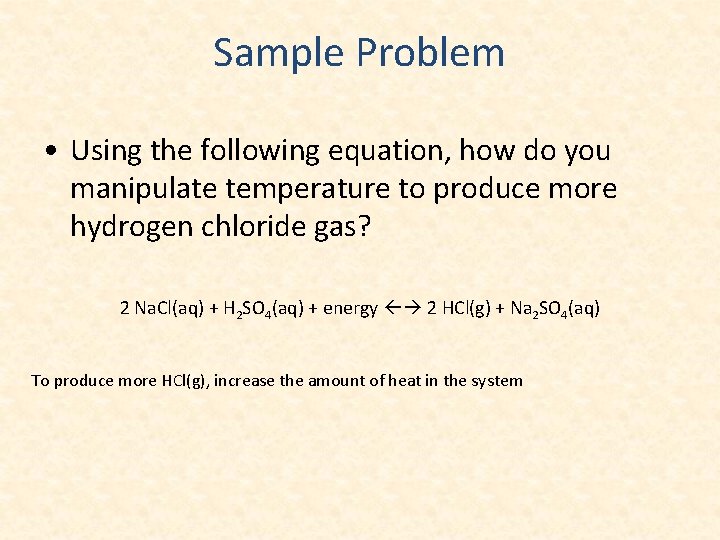
Sample Problem • Using the following equation, how do you manipulate temperature to produce more hydrogen chloride gas? 2 Na. Cl(aq) + H 2 SO 4(aq) + energy 2 HCl(g) + Na 2 SO 4(aq) To produce more HCl(g), increase the amount of heat in the system

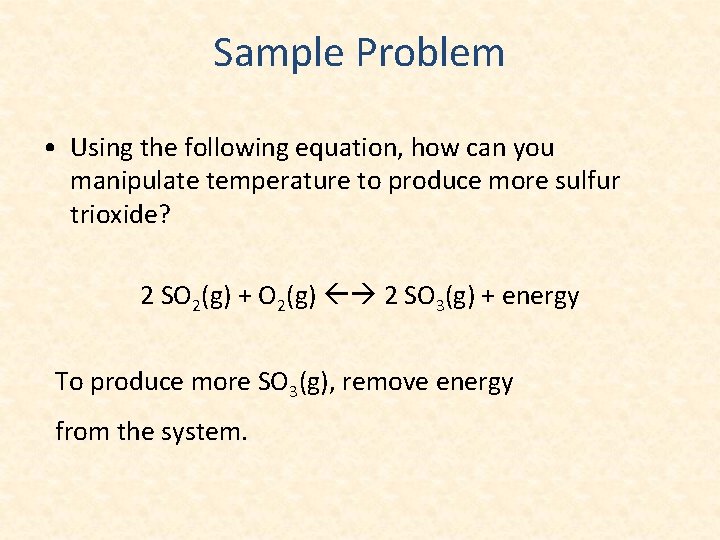
Sample Problem • Using the following equation, how can you manipulate temperature to produce more sulfur trioxide? 2 SO 2(g) + O 2(g) 2 SO 3(g) + energy To produce more SO 3(g), remove energy from the system.

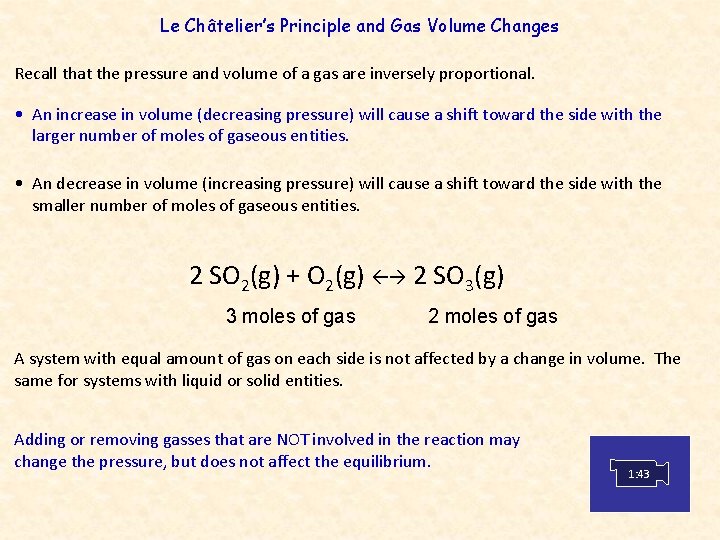
Le Châtelier’s Principle and Gas Volume Changes Recall that the pressure and volume of a gas are inversely proportional. • An increase in volume (decreasing pressure) will cause a shift toward the side with the larger number of moles of gaseous entities. • An decrease in volume (increasing pressure) will cause a shift toward the side with the smaller number of moles of gaseous entities. 2 SO 2(g) + O 2(g) 2 SO 3(g) 3 moles of gas 2 moles of gas A system with equal amount of gas on each side is not affected by a change in volume. The same for systems with liquid or solid entities. Adding or removing gasses that are NOT involved in the reaction may change the pressure, but does not affect the equilibrium. 1: 43

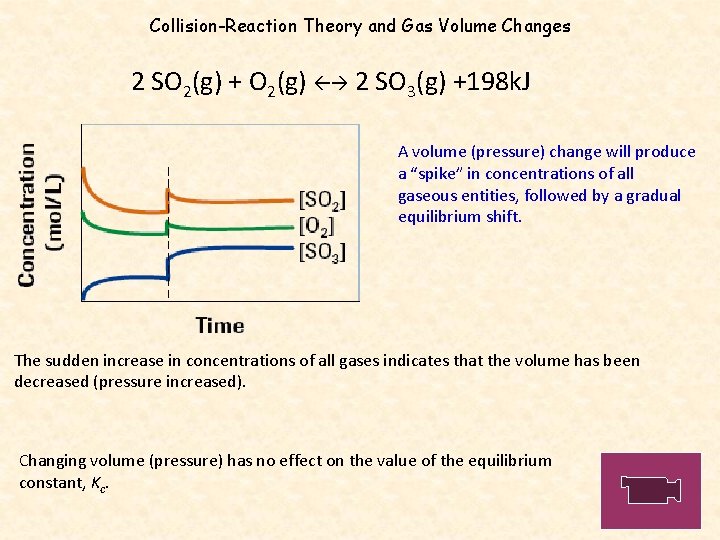
Collision-Reaction Theory and Gas Volume Changes 2 SO 2(g) + O 2(g) 2 SO 3(g) +198 k. J A volume (pressure) change will produce a “spike” in concentrations of all gaseous entities, followed by a gradual equilibrium shift. The sudden increase in concentrations of all gases indicates that the volume has been decreased (pressure increased). Changing volume (pressure) has no effect on the value of the equilibrium constant, Kc.

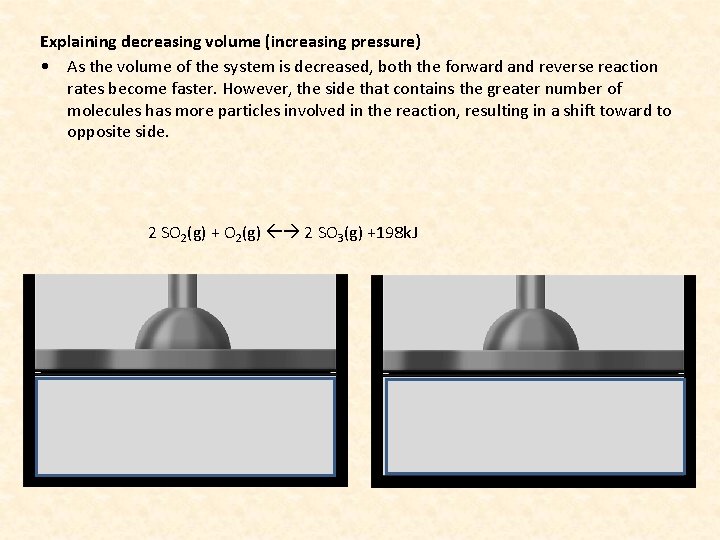
Explaining decreasing volume (increasing pressure) • As the volume of the system is decreased, both the forward and reverse reaction rates become faster. However, the side that contains the greater number of molecules has more particles involved in the reaction, resulting in a shift toward to opposite side. 2 SO 2(g) + O 2(g) 2 SO 3(g) +198 k. J

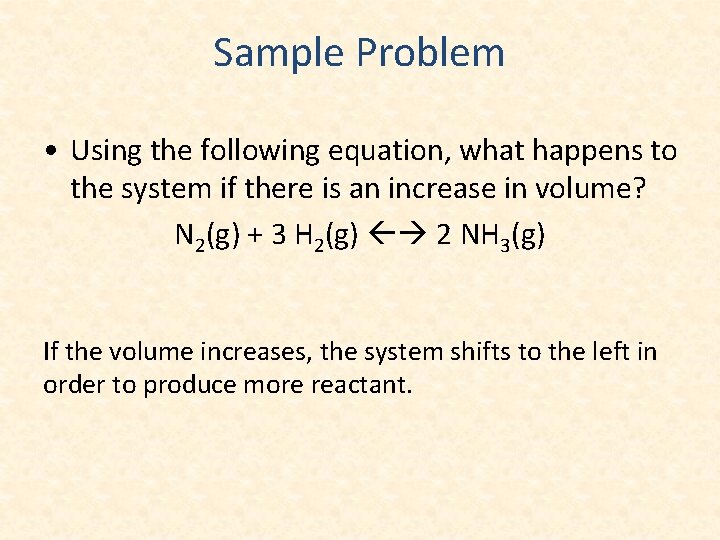
Sample Problem • Using the following equation, what happens to the system if there is an increase in volume? N 2(g) + 3 H 2(g) 2 NH 3(g) If the volume increases, the system shifts to the left in order to produce more reactant.

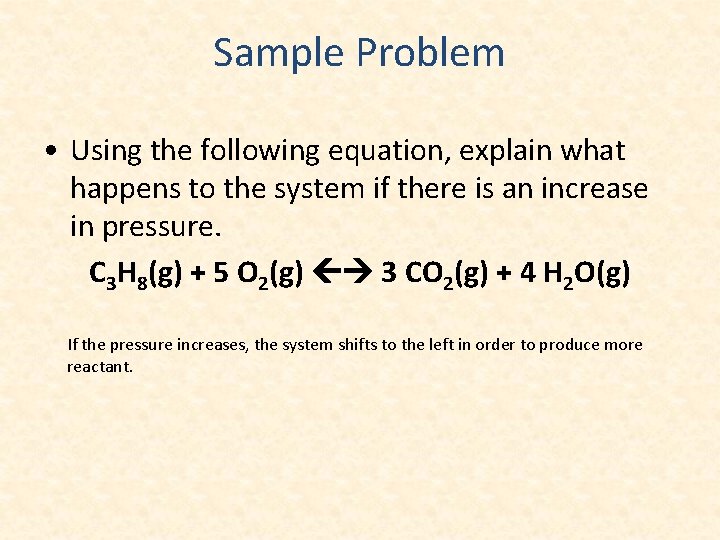
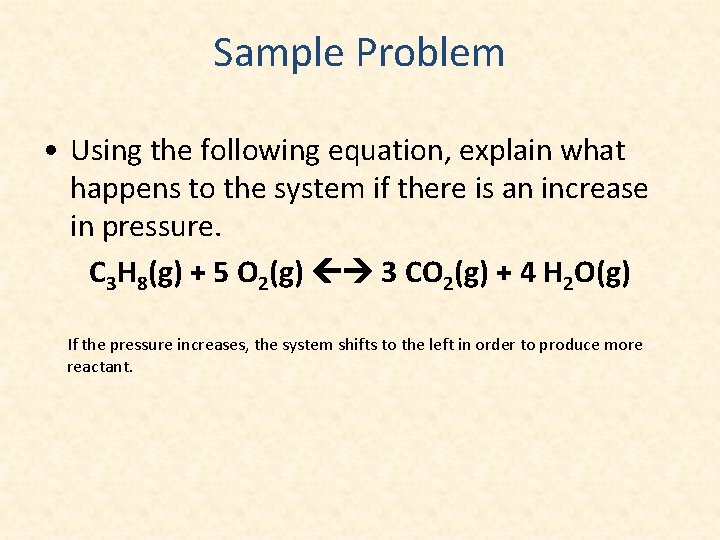
Sample Problem • Using the following equation, explain what happens to the system if there is an increase in pressure. C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) If the pressure increases, the system shifts to the left in order to produce more reactant.

Sample Problem • Using the following equation, explain what happens to the system if there is an increase in pressure. C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) If the pressure increases, the system shifts to the left in order to produce more reactant.

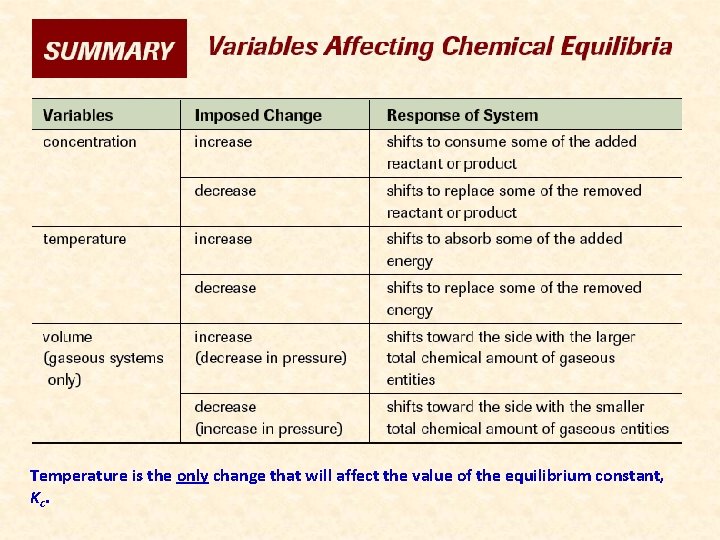
Temperature is the only change that will affect the value of the equilibrium constant, Kc.

Summary • http: //www. mhhe. com/physsci/chemistry/ess entialchemistry/flash/lechv 17. swf?

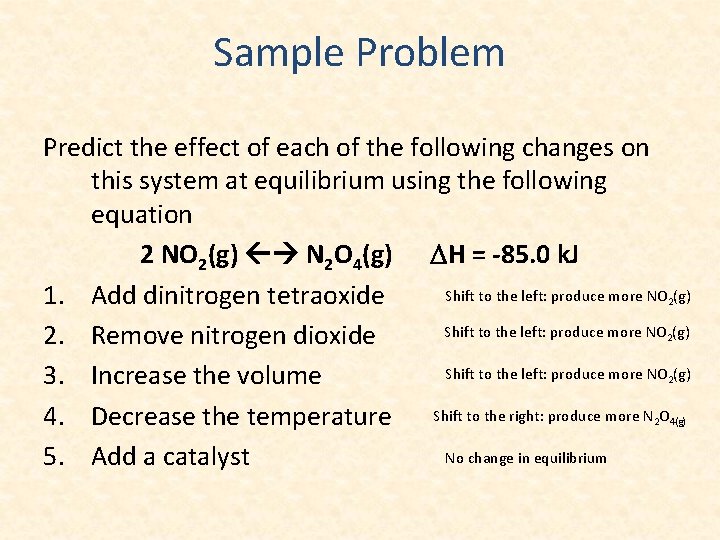
Sample Problem Predict the effect of each of the following changes on this system at equilibrium using the following equation 2 NO 2(g) N 2 O 4(g) DH = -85. 0 k. J Shift to the left: produce more NO (g) 1. Add dinitrogen tetraoxide Shift to the left: produce more NO (g) 2. Remove nitrogen dioxide Shift to the left: produce more NO (g) 3. Increase the volume 4. Decrease the temperature Shift to the right: produce more N O No change in equilibrium 5. Add a catalyst 2 2 4(g)

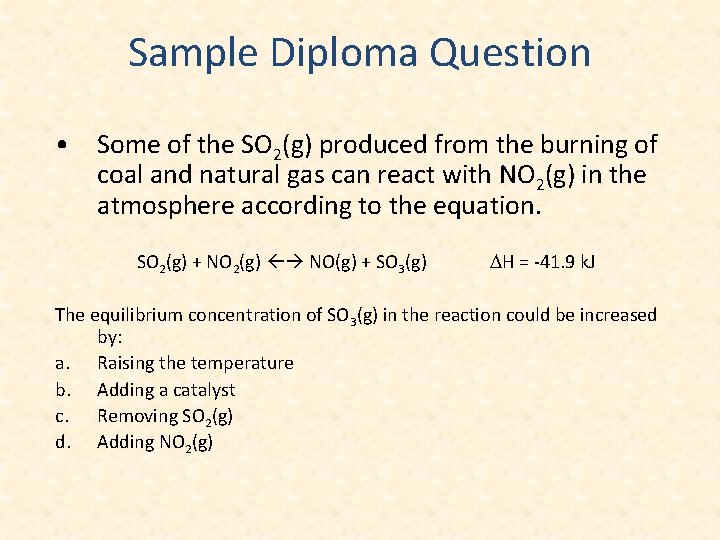
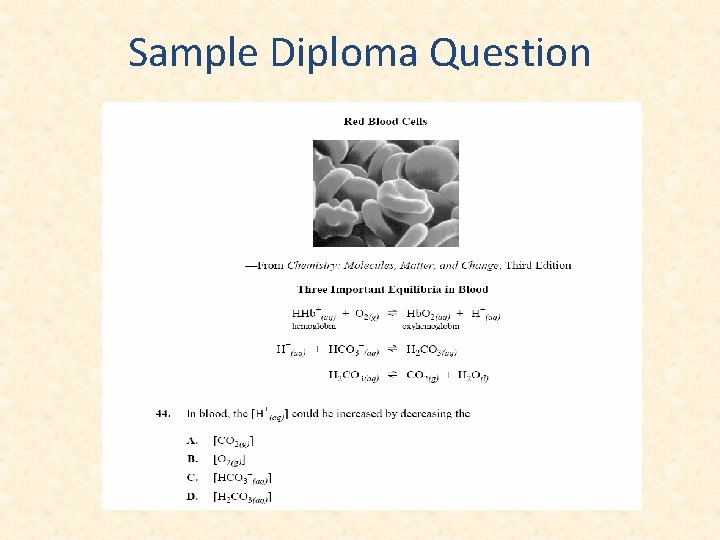
Sample Diploma Question • Some of the SO 2(g) produced from the burning of coal and natural gas can react with NO 2(g) in the atmosphere according to the equation. SO 2(g) + NO 2(g) NO(g) + SO 3(g) DH = -41. 9 k. J The equilibrium concentration of SO 3(g) in the reaction could be increased by: a. Raising the temperature b. Adding a catalyst c. Removing SO 2(g) d. Adding NO 2(g)

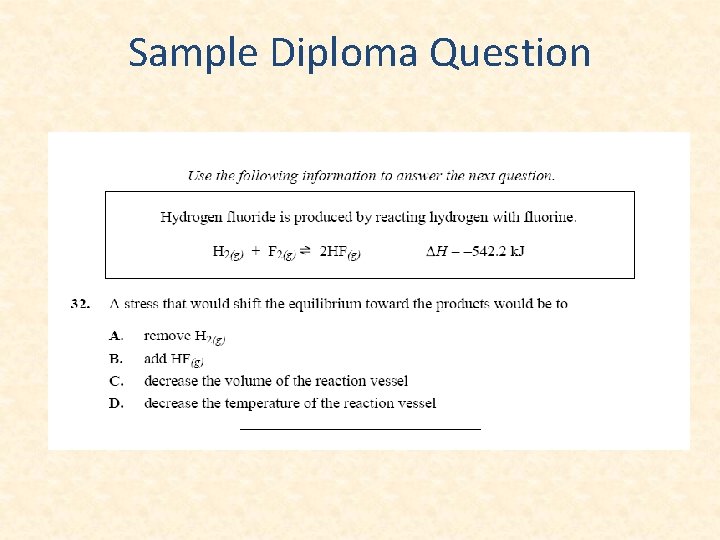
Sample Diploma Question

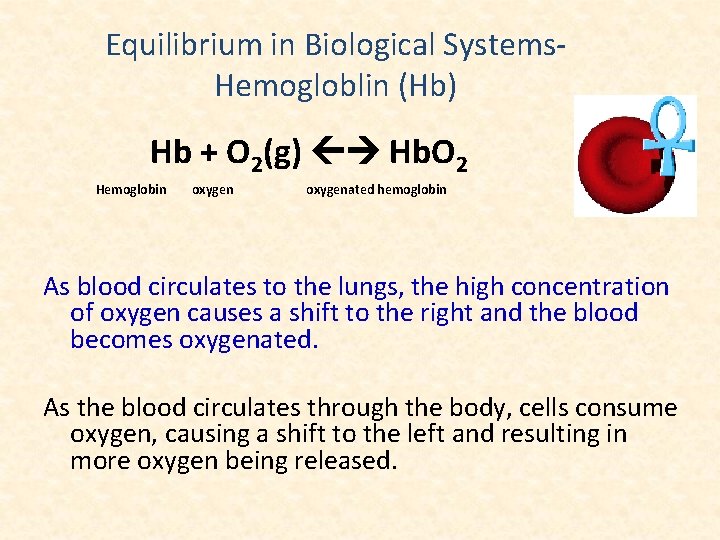
Equilibrium in Biological Systems. Hemogloblin (Hb) Hb + O 2(g) Hb. O 2 Hemoglobin oxygenated hemoglobin As blood circulates to the lungs, the high concentration of oxygen causes a shift to the right and the blood becomes oxygenated. As the blood circulates through the body, cells consume oxygen, causing a shift to the left and resulting in more oxygen being released.

Sample Diploma Question

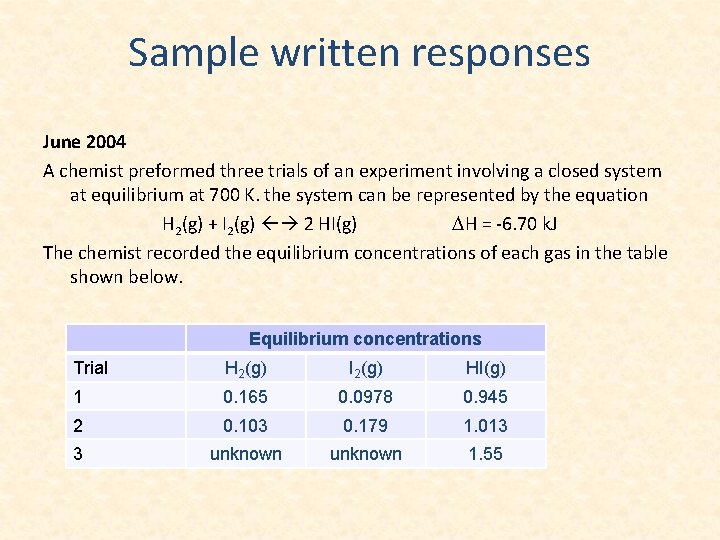
Sample written responses June 2004 A chemist preformed three trials of an experiment involving a closed system at equilibrium at 700 K. the system can be represented by the equation H 2(g) + I 2(g) 2 HI(g) DH = -6. 70 k. J The chemist recorded the equilibrium concentrations of each gas in the table shown below. Equilibrium concentrations Trial H 2(g) I 2(g) HI(g) 1 0. 165 0. 0978 0. 945 2 0. 103 0. 179 1. 013 3 unknown 1. 55

1 a. Provide the equilibrium constant expression for the formation of HI(g) 1 b. Using the information from trials 1 and 2 to calculate an average value for the equilibrium constant , Keq, for the formation of HI(g).

1 c. In trial 3, the equilibrium was established by allowing a sample of HI(g) to decompose. Calculate the equilibrium concentration of H 2(g) and I 2(g) for trial 3.

1 d. Identify a stress that would increase the value of the equilibrium constant and explain how the stress is responsible for the increase.

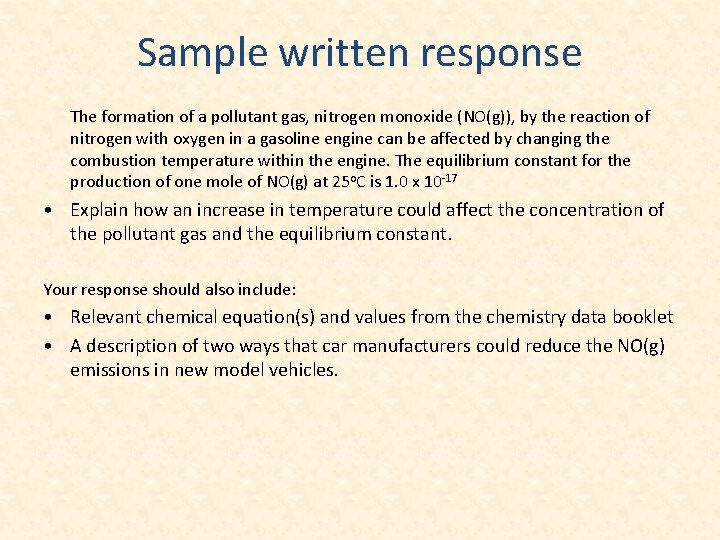
Sample written response The formation of a pollutant gas, nitrogen monoxide (NO(g)), by the reaction of nitrogen with oxygen in a gasoline engine can be affected by changing the combustion temperature within the engine. The equilibrium constant for the production of one mole of NO(g) at 25 o. C is 1. 0 x 10 -17 • Explain how an increase in temperature could affect the concentration of the pollutant gas and the equilibrium constant. Your response should also include: • Relevant chemical equation(s) and values from the chemistry data booklet • A description of two ways that car manufacturers could reduce the NO(g) emissions in new model vehicles.


ü Read pgs. 690 – 695 ü pg. 695 Practice #’s 1 – 3 ü pg. 699 Section 15. 2 Questions #’s 1 – 7 ü Note: 2 b = refer to chem. 20 bonding unit (chapter 3) ü Do case study questions 1 and 2 on p. 697
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chemical formulas and chemical compounds chapter 7 review
Chemical formulas and chemical compounds chapter 7 review Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical oxygen demand principle
Chemical oxygen demand principle Wordsworth definition of poetry
Wordsworth definition of poetry How did postema feel about sexist remarks from spectators
How did postema feel about sexist remarks from spectators Context evaluation of curriculum
Context evaluation of curriculum Radial pulse range
Radial pulse range Regular vital signs
Regular vital signs Henderson's 14 components
Henderson's 14 components Chickering's 7 vectors
Chickering's 7 vectors Mass media audience
Mass media audience According to jung the unconscious mind is characterized by
According to jung the unconscious mind is characterized by Theme of the world according to humphrey
Theme of the world according to humphrey Sir philip sidney defense of poesy
Sir philip sidney defense of poesy According to synonym
According to synonym What are the consequences of sin in genesis chapter 3
What are the consequences of sin in genesis chapter 3 Leonard bloomfield contribution to linguistics
Leonard bloomfield contribution to linguistics Definition of school health
Definition of school health Adaptation psychology piaget
Adaptation psychology piaget Mental health nursing definition according to who
Mental health nursing definition according to who Steel rods and pipes on which forms are lifted
Steel rods and pipes on which forms are lifted Care plan on ocd
Care plan on ocd According to the third law of motion action and reaction
According to the third law of motion action and reaction Hand washing steps nabh
Hand washing steps nabh Chester irving barnard
Chester irving barnard Contemporary psychodynamic theories
Contemporary psychodynamic theories Definition of leadership
Definition of leadership Relational concept examples
Relational concept examples Disaster scenario media and information literacy
Disaster scenario media and information literacy Importance of health education
Importance of health education