Le Chteliers Principle Chapter 15 Le Chteliers Principle











- Slides: 16

Le Châtelier’s Principle Chapter 15

Le Châtelier’s Principle “If a system at equilibrium is disturbed by a change in temperature, pressure, or the concentration of one of the components, the system will shift its equilibrium position so as to counteract the effect of the disturbance. ” –When conditions of equilibrium are altered, the equilibrium shifts in the direction that minimizes or reduces the effect of the change until a new state of balance is attained.

Change in concentration • Changing the concentration of a reactant or product, impacts the Q value for the reaction • When a system at equilibrium has an increase in concentration of a substance (reactant or product), the system reacts to consume some of the substance. • If we decrease the concentration of a substance, the system reacts to produce some more of that substance.

N 2(g)+ 3 H 2(g) ↔ 2 NH 3(g) • What happens when you add H 2? – System would try to consume H 2, so shift would be to right to make more product • What happens when you add more NH 3? – System would try to reduce NH 3, by making more nitrogen and hydrogen gas, shift to left • What happens when you remove N 2? – System would try to make more N 2, shift would be to left

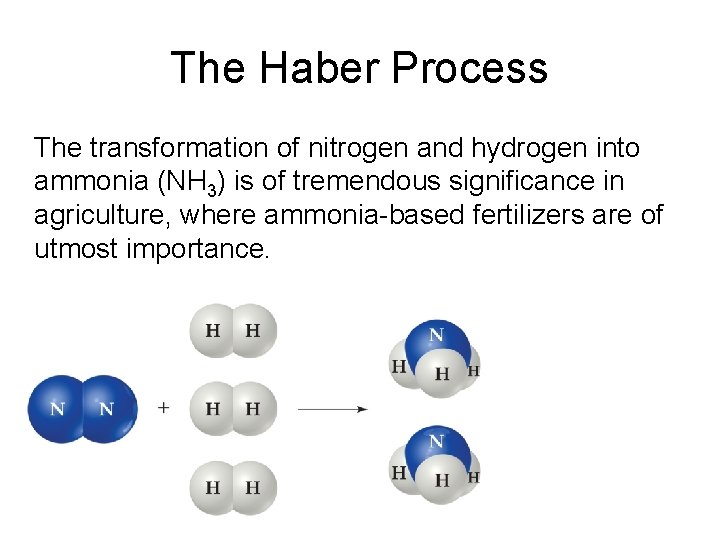
The Haber Process The transformation of nitrogen and hydrogen into ammonia (NH 3) is of tremendous significance in agriculture, where ammonia-based fertilizers are of utmost importance.

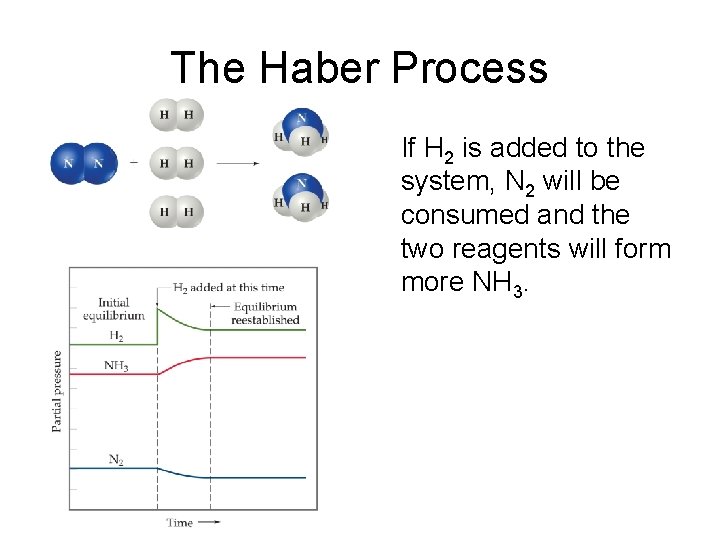
The Haber Process If H 2 is added to the system, N 2 will be consumed and the two reagents will form more NH 3.

Changing the Volume at Equilibrium • According to Boyle’s Law, a decrease in volume increases the pressure of the system – Le Chatelier’s principle states that the system will respond by shifting its equilibrium position to reduce the pressure • To reduce the pressure, you must decrease the number of gas molecules in system • At constant temperature, reducing the volume of a gaseous equilibrium mixture causes the system to shift in the direction that reduces the number of moles of gas. – Increasing the volume causes a shift in the direction that produces more gas molecules

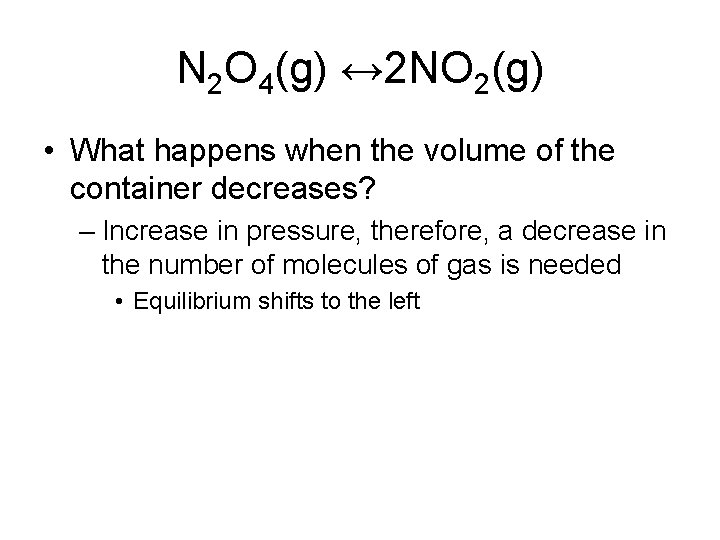
N 2 O 4(g) ↔ 2 NO 2(g) • What happens when the volume of the container decreases? – Increase in pressure, therefore, a decrease in the number of molecules of gas is needed • Equilibrium shifts to the left

N 2(g) + 3 H 2(g) ↔ 2 NH 3(g) • What would happen if the pressure of this system was increased? – Decrease in volume, so fewer molecules are needed at equilibrium – 4 moles reactant > 2 moles of product – Shift is to the right

Effect of Temperature Changes • Changes in concentration and partial pressures cause shifts in equilibrium without changing the value of K • In an endothermic (heat absorbing) reaction, heat is considered a reactant, whereas, in an exothermic reaction, heat is a product

reactants + heat ↔ products • When the temperature of the system at equilibrium is increased, the system reacts as if we added a reactant to an endothermic reaction or a product to an exothermic reaction. – Equilibrium shifts in the direction that consumes the excess reactant (or product), namely heat. • Equilibrium shifts to the right • K increases for the reaction

Reactants ↔ products + heat • Exothermic reaction – Heat is a product – Adding heat shifts equilibrium to left • Decreases value of K

Heat + A ↔ B • What would happen to equilibrium if the reaction was cooled? – Cooling is the removal of heat (like losing a reactant for this reaction) – Equilibrium shifts to the left

Dilution • Dilutions decrease the concentration of a reactant or product – Acts the same as decreasing that concentration on the equilibrium of the system

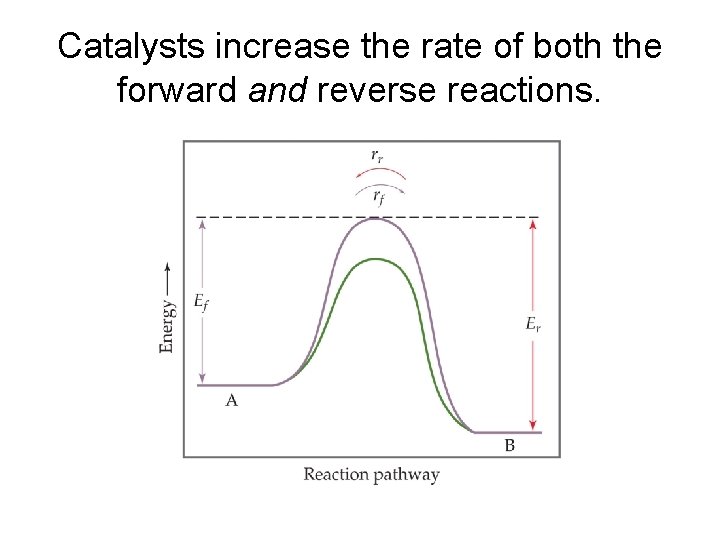
Catalysts increase the rate of both the forward and reverse reactions.

Equilibrium is achieved faster, but the equilibrium composition remains unaltered. Catalyst only impacts the activation energy and rate.