A Thistlewood 2007 Organic nomenclature educate net 2007














- Slides: 28

© A. Thistlewood 2007 Organic nomenclature © e-ducate. net 2007

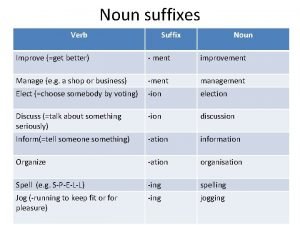
© A. Thistlewood 2007 What is it? • The systematic naming of an organic compound generally requires the identification and naming of a parent structure. • This name may then be modified by prefixes, infixes, and, in the case of a parent hydride, suffixes, which give the precise structural changes required to generate the actual compound from the parent structure. © e-ducate. net 2007

© A. Thistlewood 2007 Naming straight-chain alkanes • Naming of straight chain alkanes (alkanes that do not branch) is a straightforward process. • To give an alkane a name, a prefix indicating the number of carbons in the molecule is added to the suffix ane, identifying both the kind of molecule (an alkane) and how many carbons the molecule has (the prefix). • The name pentane, for example, tells you that the molecule is an alkane (thus the ane ending) and that it has five carbons (pent indicates five). © e-ducate. net 2007

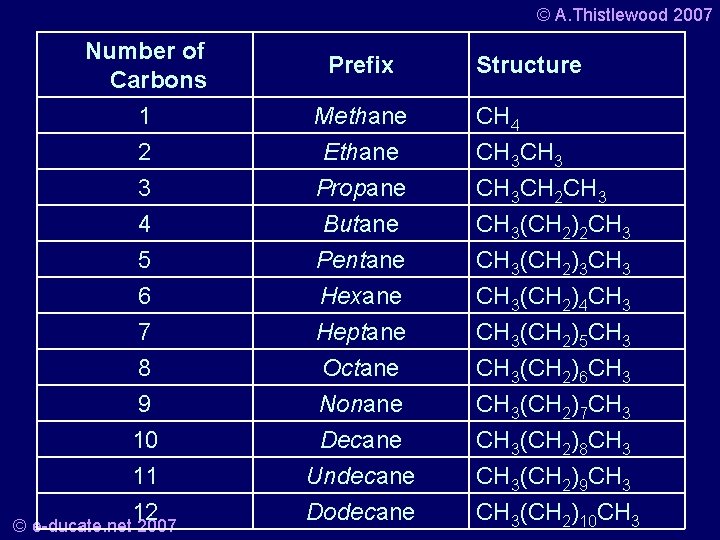
© A. Thistlewood 2007 • Prefixes for alkanes that have 1 -4 carbons are rooted historically. • These are methane, propane, and butane, respectively. • On the other hand, for 5 carbons and up a prefix derived from greek is given. (An easy way to remember the first four names is the anagram Mary eats peanut butter, standing for methane, propane, butane). • Learning the prefixes for up to twelve carbons is a good idea. © e-ducate. net 2007

© A. Thistlewood 2007 Number of Carbons 1 2 Methane Ethane 3 4 5 6 7 8 9 10 11 12 Propane Butane Pentane Hexane Heptane Octane Nonane Decane Undecane Dodecane © e-ducate. net 2007 Prefix Structure CH 4 CH 3 CH 2 CH 3(CH 2)3 CH 3(CH 2)4 CH 3(CH 2)5 CH 3(CH 2)6 CH 3(CH 2)7 CH 3(CH 2)8 CH 3(CH 2)9 CH 3(CH 2)10 CH 3

© A. Thistlewood 2007 Naming branched alkanes • The nomenclature becomes more complex if the alkane branches. In such a case, there are several rules that you must follow to give the alkane the correct name. • Find the longest chain of carbons in the molecule. The number of carbons in the longest chain becomes the parent name. • After finding the parent chain, you number the parent chain starting with the end nearest the first substituent (a substituent is any fragment that juts off the main © e-ducate. net 2007 chain).

© A. Thistlewood 2007 • Next, determine the names of all substituents. Substituents are named as if the piece were a separate molecule, except that the suffix of yl is used rather than ane. • Thus, a two-carbon substituent would be an ethyl substituent (not an ethane substituent). • Put the substituents in alphabetical order (ie. ethyl before methyl) in front of the parent name. • Next, identify the positions of all substituents in the name by placing the carbon number where the substituent attaches to the parent chain in front of it. For example, 2© e-ducate. net 2007 methylheptane indicates that a methyl

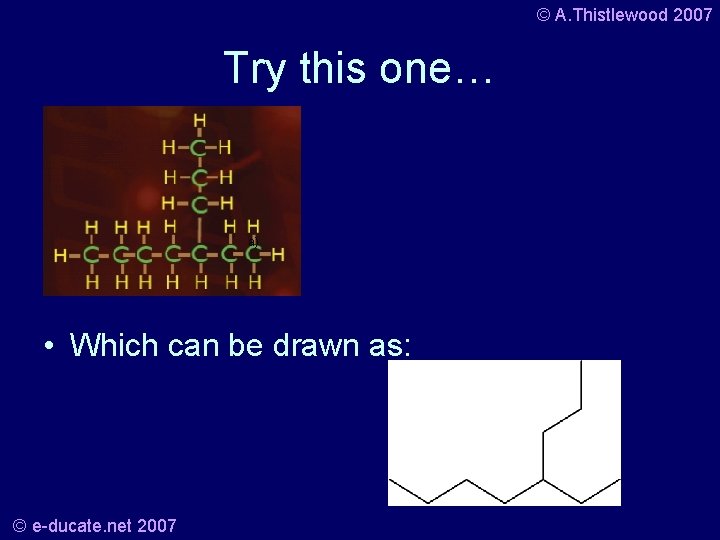
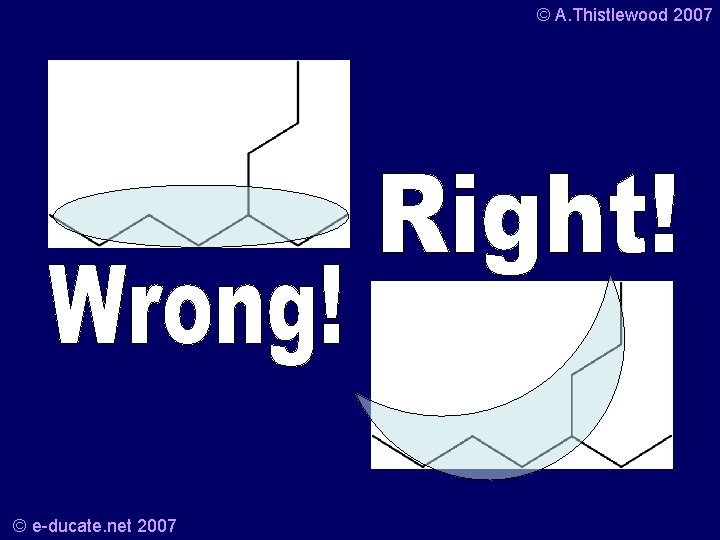
© A. Thistlewood 2007 Try this one… a) • Which can be drawn as: © e-ducate. net 2007

© A. Thistlewood 2007 Step 1 • Find the longest carbon chain in the molecule. – First, begin by finding the parent chain in the molecule--that is, the longest possible chain of connecting carbons. – Note that the parent chain is not necessarily the chain that simply follows from left to right. – For example, if you were to count the number of carbons directly from left to right in this molecule you would get 7 carbons. This is not the parent chain, however! – If you start at the left and then count up where the molecule branches, you find that there are 8 © e-ducate. net 2007

© A. Thistlewood 2007 © e-ducate. net 2007

© A. Thistlewood 2007 Step 2 • Number the parent chain. – The second step is to number the carbons in the parent chain starting at the end closest to the first substituent. – It is important to number the molecule from the correct end (in other words, in this example do you number the alkane from right to left or left to right). – Following this rule, on this molecule you number from right to left, as the 2 -carbon substituent is closer to that end. © e-ducate. net 2007

© A. Thistlewood 2007 8 7 6 2 1 4 3 5 1 2 3 7 8 © e-ducate. net 2007 5 6 4

© A. Thistlewood 2007 Step 3 • Name all the substituents. – You then identify the names of the substituents. – In this case, the only substituent is a 2 carbon group at the number 4 carbon. – This is an ethyl group. 4 © e-ducate. net 2007

© A. Thistlewood 2007 Step 4 • Put the substituents in alphabetical order. – The next step is to put the substituents in alphabetical order (ie. ethyl before methyl) but since there is only one substituent this is unnecessary. © e-ducate. net 2007

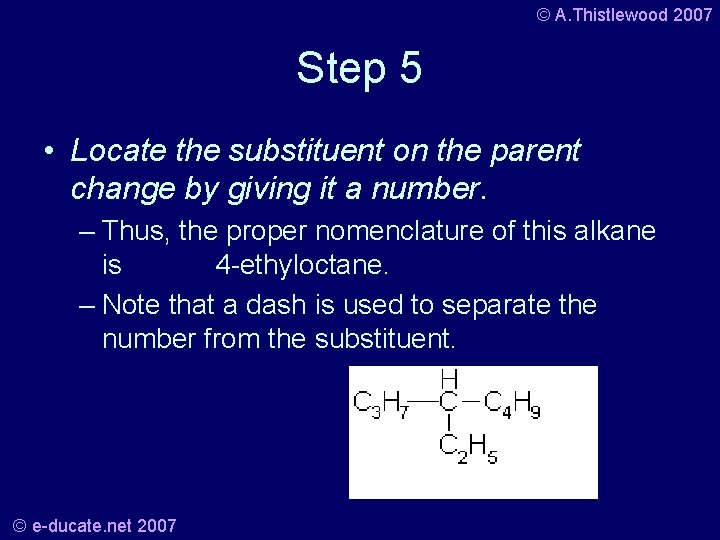
© A. Thistlewood 2007 Step 5 • Locate the substituent on the parent change by giving it a number. – Thus, the proper nomenclature of this alkane is 4 -ethyloctane. – Note that a dash is used to separate the number from the substituent. © e-ducate. net 2007

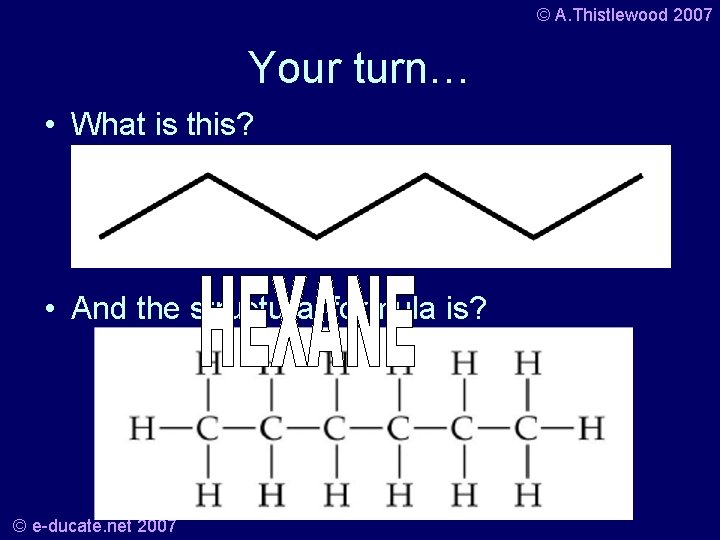
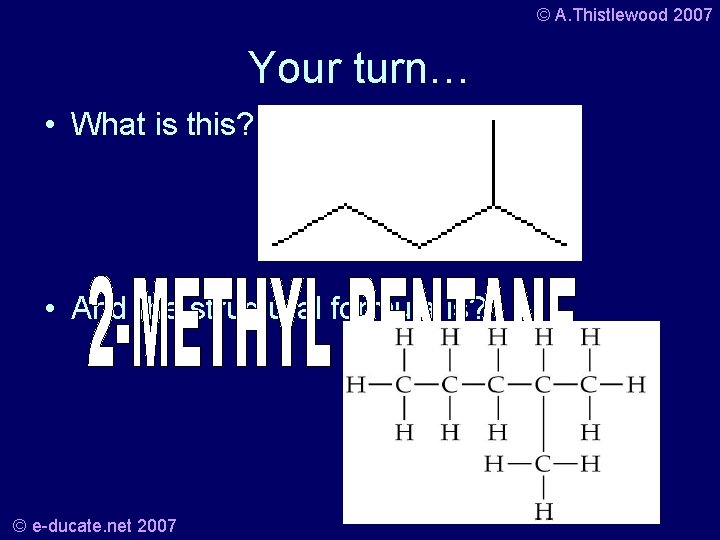
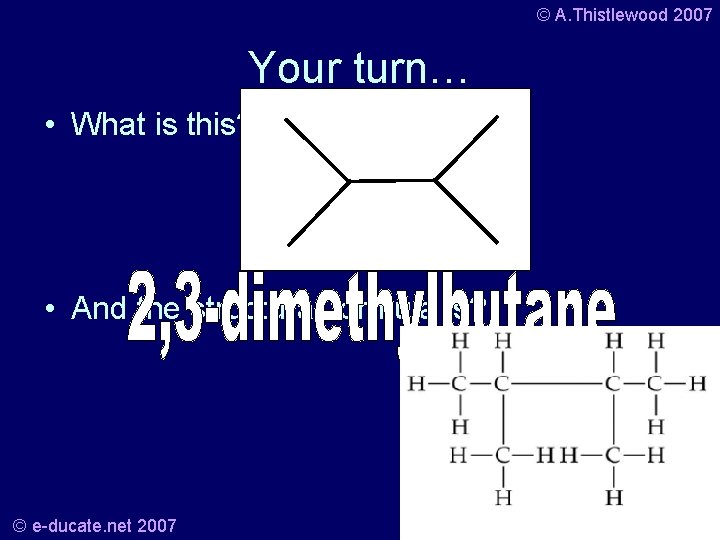
© A. Thistlewood 2007 Your turn… • What is this? • And the structural formula is? © e-ducate. net 2007

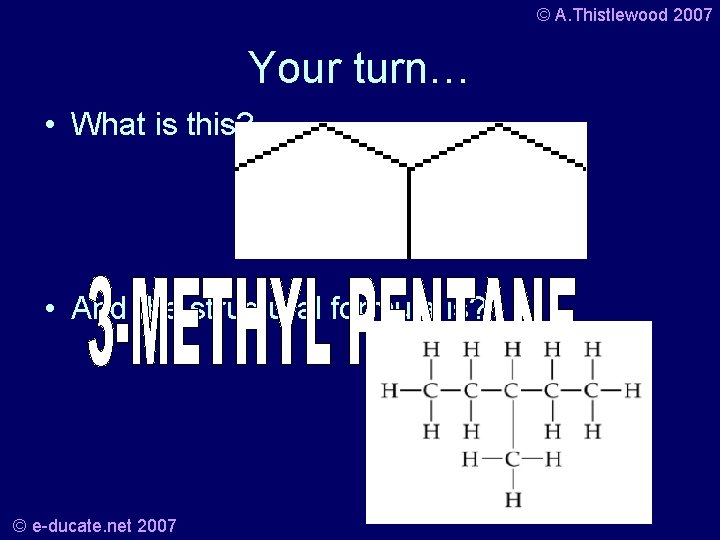
© A. Thistlewood 2007 Your turn… • What is this? • And the structural formula is? © e-ducate. net 2007

© A. Thistlewood 2007 Your turn… • What is this? • And the structural formula is? © e-ducate. net 2007

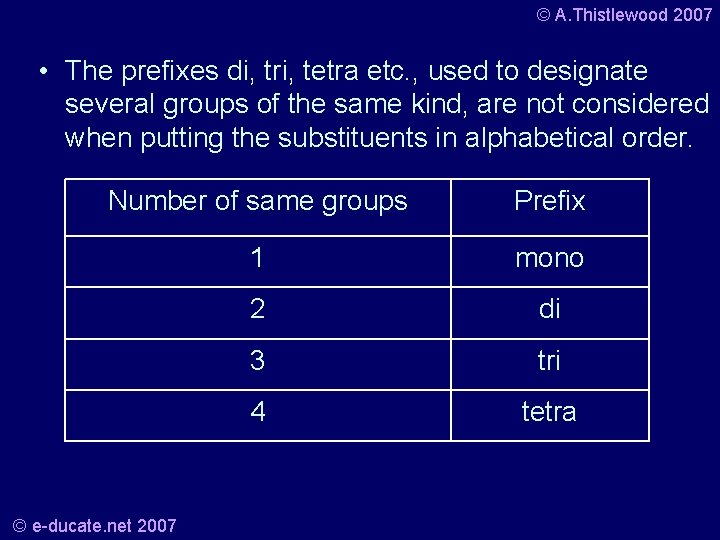
© A. Thistlewood 2007 • The prefixes di, tri, tetra etc. , used to designate several groups of the same kind, are not considered when putting the substituents in alphabetical order. Number of same groups Prefix 1 mono 2 di 3 tri 4 tetra © e-ducate. net 2007

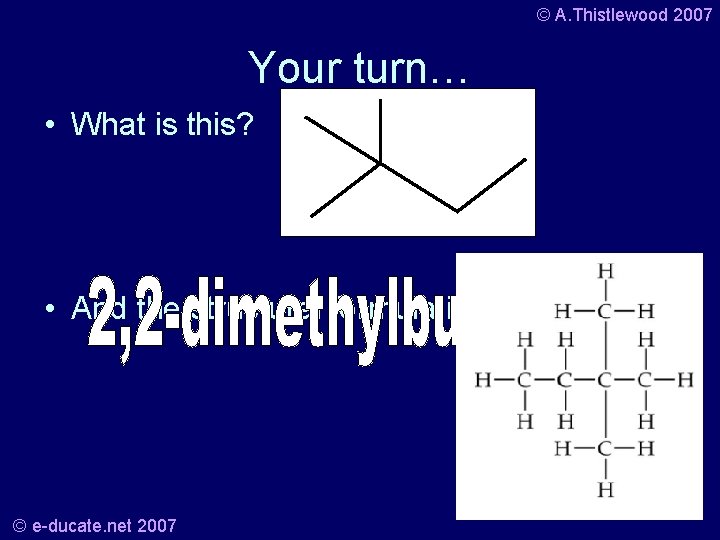
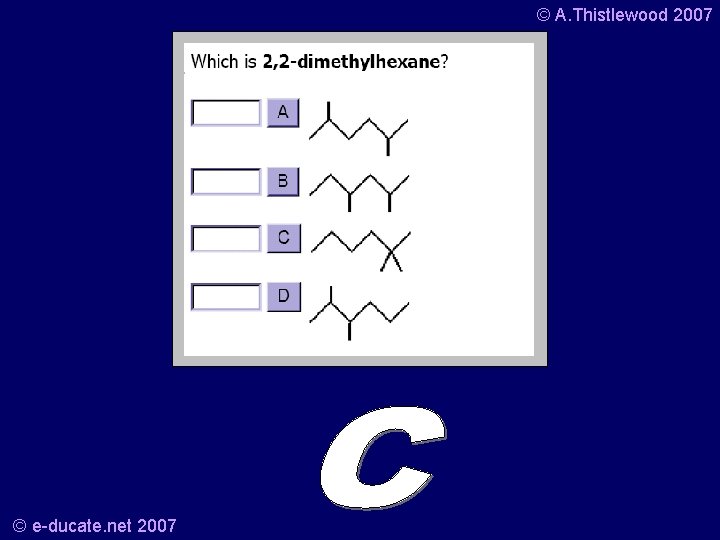
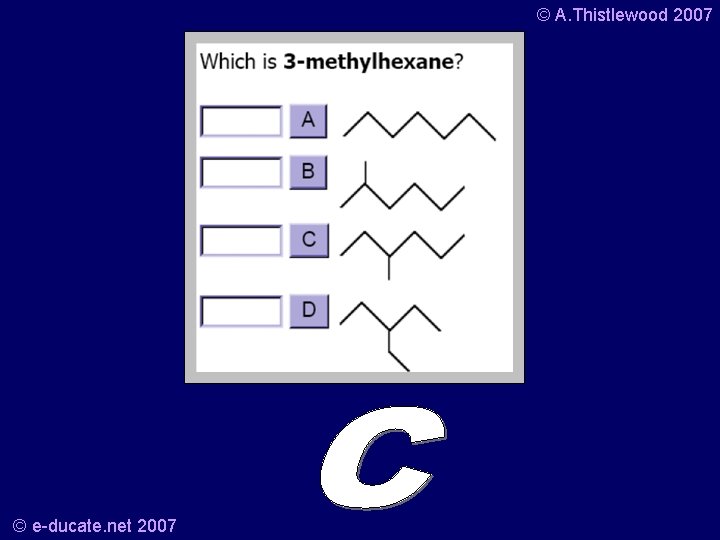
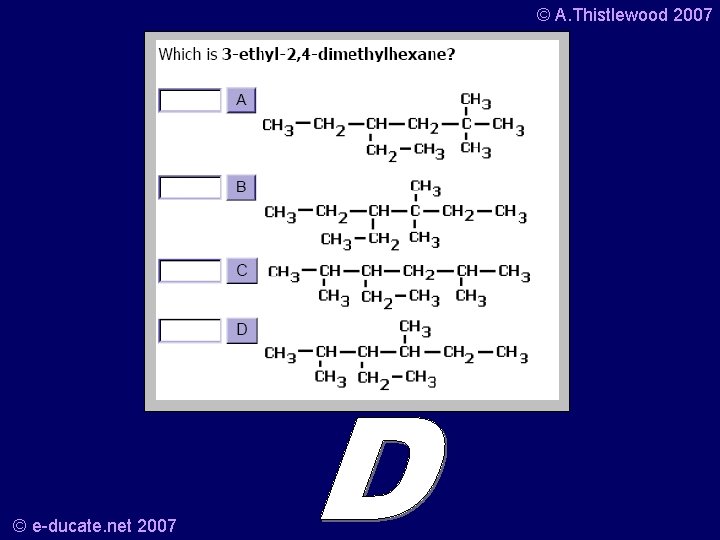
© A. Thistlewood 2007 Your turn… • What is this? • And the structural formula is? © e-ducate. net 2007

© A. Thistlewood 2007 Your turn… • What is this? • And the structural formula is? © e-ducate. net 2007

© A. Thistlewood 2007 © e-ducate. net 2007

© A. Thistlewood 2007 © e-ducate. net 2007

© A. Thistlewood 2007 © e-ducate. net 2007

© A. Thistlewood 2007 © e-ducate. net 2007

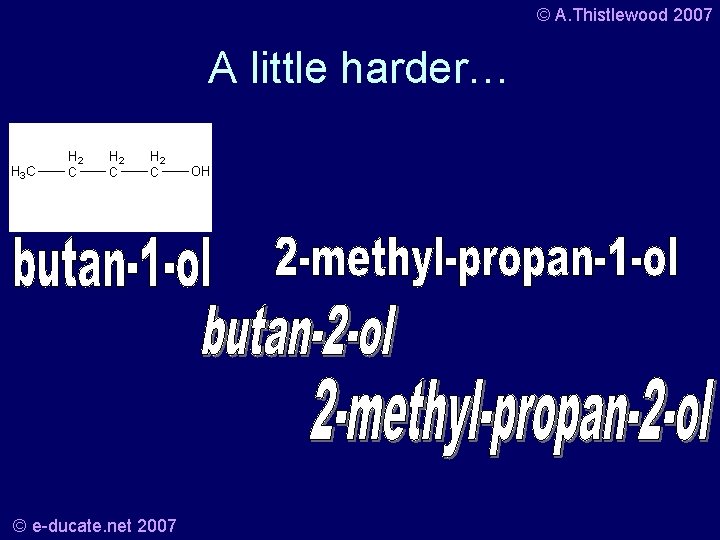
© A. Thistlewood 2007 A little harder… • Butanol… C 4 H 10 O – What about these 4 structures? – How many C, H, O? – And what are they all called? © e-ducate. net 2007

© A. Thistlewood 2007 A little harder… © e-ducate. net 2007

© A. Thistlewood 2007 Homework… • Draw and name all the possible structures with the formula: 1. C 3 H 7 Br (Br = bromo) 2. C 5 H 12 • Draw the following compounds: 1. 3 -ethyl-2, 4 -dimethylheptane 2. 2, 2, 4 -trimethylpentane 3. 4 -ethyl-3, 5 -dimethylheptane © e-ducate. net 2007
 Iupac
Iupac Basic organic nomenclature packet
Basic organic nomenclature packet Iupac nomenclature of organic chemistry
Iupac nomenclature of organic chemistry Organic chemistry nomenclature
Organic chemistry nomenclature Belmayne educate together
Belmayne educate together Derivation example
Derivation example Educate empower evolve
Educate empower evolve It takes the whole village to educate a child
It takes the whole village to educate a child Educate adj
Educate adj Educate your desires
Educate your desires Inform educate and empower public health
Inform educate and empower public health Change 3.hali
Change 3.hali Aws vocareum
Aws vocareum Educate women
Educate women Noun of improve
Noun of improve Educate fortox
Educate fortox Yonatan shemmer
Yonatan shemmer Explain the social impact of ict
Explain the social impact of ict It takes a whole village to educate a child
It takes a whole village to educate a child Kilcolgan educate together
Kilcolgan educate together Plastic fingerprints
Plastic fingerprints T. trimpe 2007 http //sciencespot.net/
T. trimpe 2007 http //sciencespot.net/ T trimpe 2003 http sciencespot net crossword answers
T trimpe 2003 http sciencespot net crossword answers Ado.net vb.net
Ado.net vb.net Achmed lach net ich krieg mein tach net
Achmed lach net ich krieg mein tach net Acid nomenclature
Acid nomenclature General molecular formula for alkene
General molecular formula for alkene Elastic energy
Elastic energy Simple key loader (skl) basic usage
Simple key loader (skl) basic usage