9182021 Reactivity series 9182021 Reactions of metals with




- Slides: 8

9/18/2021 Reactivity series

9/18/2021 Reactions of metals with oxygen When a metal reacts with oxygen it will form a METAL OXIDE. This is what happens when a metal rusts. We can make this reaction happen quicker by burning the metal. METAL + OXYGEN METAL OXIDE Copy and complete the following reactions: 1) Magnesium + oxygen 2) Copper + oxygen 3) Calcium + oxygen 4) Iron + oxygen

9/18/2021 Reactions of metals with water When a metal reacts with water hydrogen is always given off. The other product will be either a metal hydroxide or a metal oxide. METAL + WATER METAL OXIDE + HYDROGEN METAL HYDROXIDE + HYDROGEN Copy and complete the following reactions: 1) Sodium + water 2) Potassium + water 3) Calcium + water 4) Iron + steam

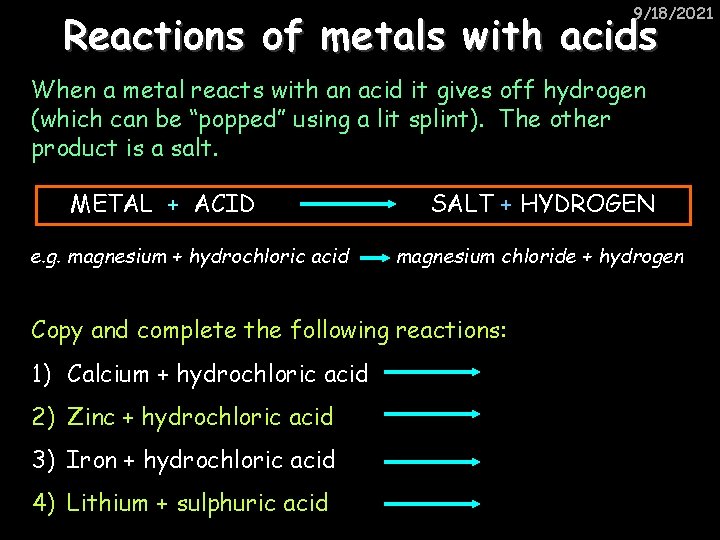
9/18/2021 Reactions of metals with acids When a metal reacts with an acid it gives off hydrogen (which can be “popped” using a lit splint). The other product is a salt. METAL + ACID e. g. magnesium + hydrochloric acid SALT + HYDROGEN magnesium chloride + hydrogen Copy and complete the following reactions: 1) Calcium + hydrochloric acid 2) Zinc + hydrochloric acid 3) Iron + hydrochloric acid 4) Lithium + sulphuric acid

9/18/2021 Complete the following reactions: 1) Lithium + water Lithium hydroxide + hydrogen 2) Lithium + hydrochloric acid Lithium chloride + hydrogen 3) Silver + oxygen Silver oxide 4) Magnesium + sulphuric acid Magnesium sulphate + hydrogen 5) Potassium + oxygen Potassium oxide 6) Aluminium + oxygen Aluminium oxide 7) Manganese + water Manganese oxide + hydrogen 8) Sodium + sulphuric acid Sodium sulphate + hydrogen 9) Lithium + oxygen Lithium oxide 10) Nickel + hydrochloric acid Nickel chloride + hydrogen

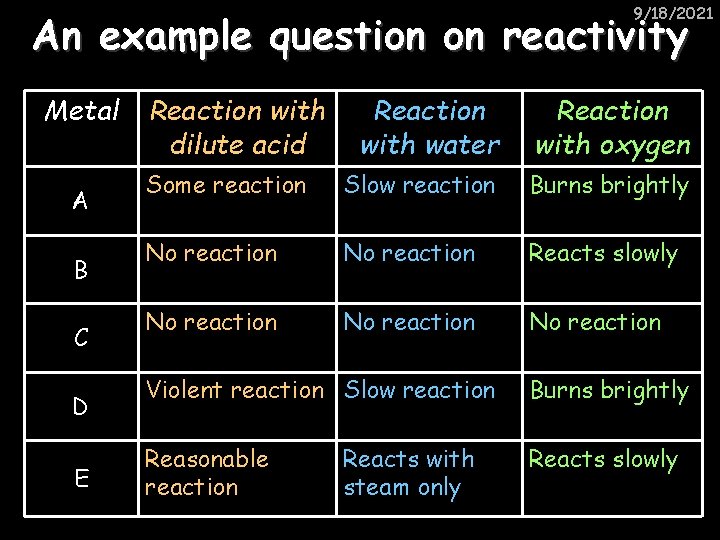
9/18/2021 An example question on reactivity Metal A B C D E Reaction with dilute acid Reaction with water Reaction with oxygen Some reaction Slow reaction Burns brightly No reaction Reacts slowly No reaction Violent reaction Slow reaction Burns brightly Reasonable reaction Reacts slowly Reacts with steam only

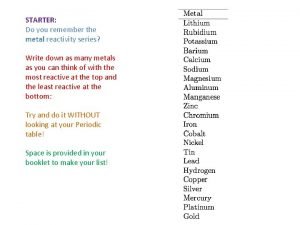
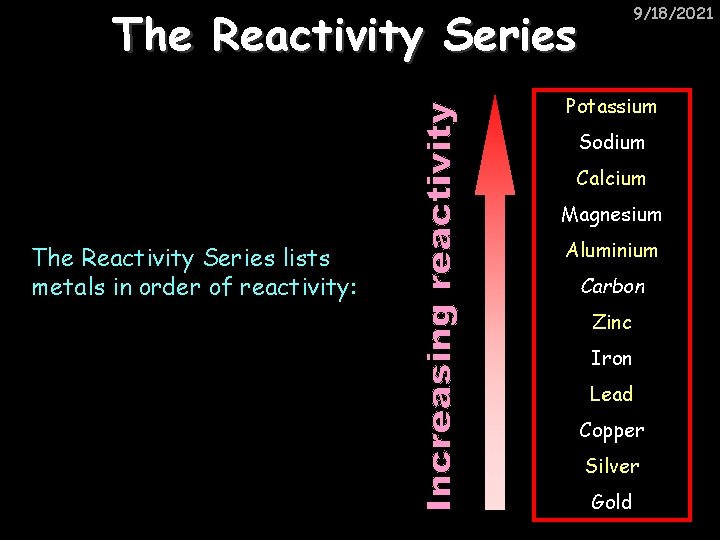
9/18/2021 The Reactivity Series Potassium Sodium Calcium Magnesium The Reactivity Series lists metals in order of reactivity: Aluminium Carbon Zinc Iron Lead Copper Silver Gold

9/18/2021 This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.