3030 Horizons in Healthcare Research NHMRC CTC 30







- Slides: 12

30/30 Horizons in Healthcare Research NHMRC CTC 30 th Anniversary Symposium 1 March 2019 The future of clinical trial funding Professor Anne Kelso AO Chief Executive Officer

Clinical trials funding in NHMRC’s new grant program

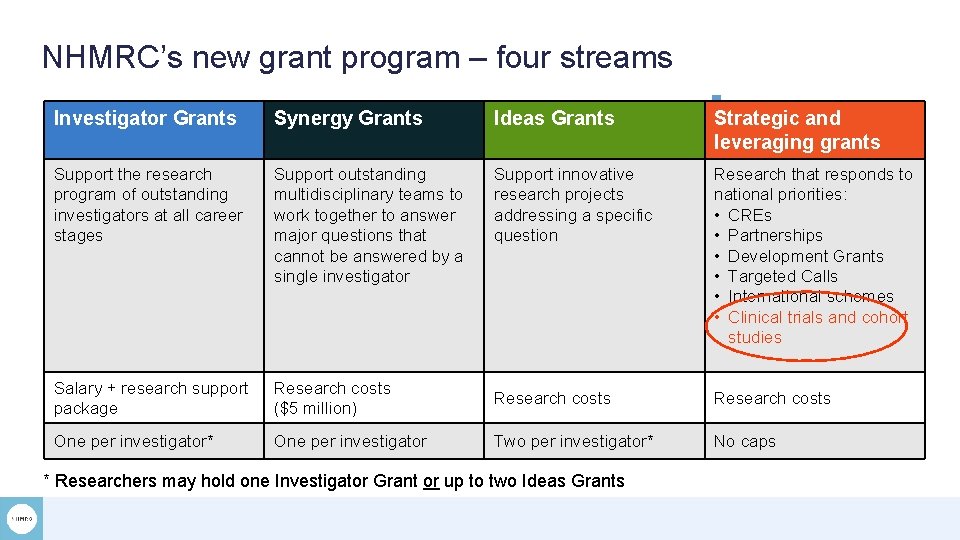
NHMRC’s new grant program – four streams Investigator Grants Synergy Grants Ideas Grants Strategic and leveraging grants Support the research program of outstanding investigators at all career stages Support outstanding multidisciplinary teams to work together to answer major questions that cannot be answered by a single investigator Support innovative research projects addressing a specific question Research that responds to national priorities: • CREs • Partnerships • Development Grants • Targeted Calls • International schemes • Clinical trials and cohort studies Salary + research support package Research costs ($5 million) Research costs One per investigator* One per investigator Two per investigator* No caps * Researchers may hold one Investigator Grant or up to two Ideas Grants

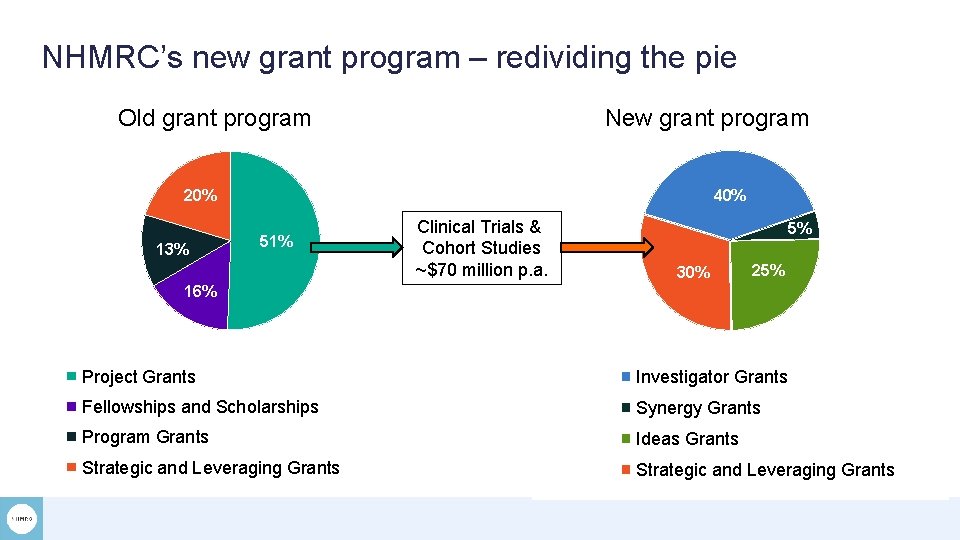
NHMRC’s new grant program – redividing the pie Old grant program New grant program 20% 13% 40% 51% Clinical Trials & Cohort Studies ~$70 million p. a. 5% 30% 25% 16% Project Grants Investigator Grants Fellowships and Scholarships Synergy Grants Program Grants Ideas Grants Strategic and Leveraging Grants

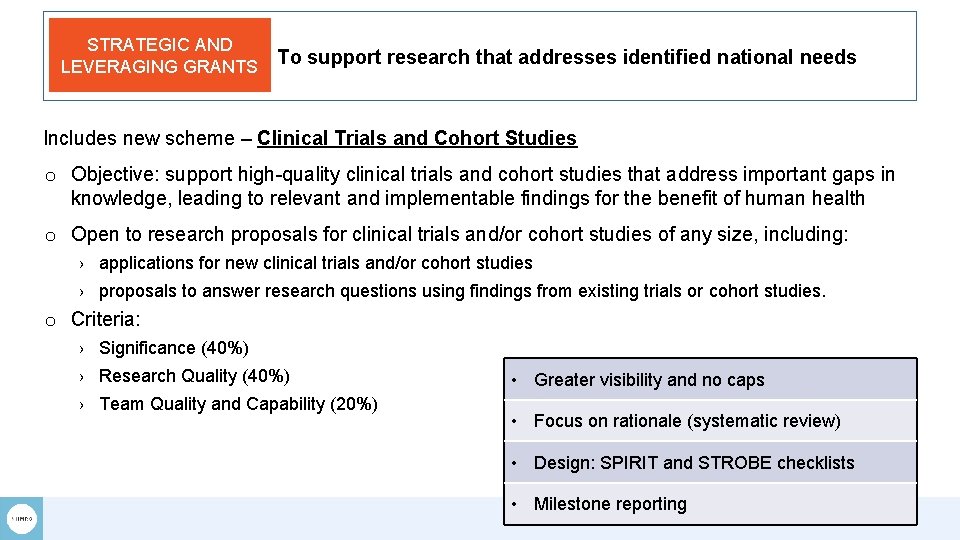
STRATEGIC AND LEVERAGING GRANTS To support research that addresses identified national needs Includes new scheme – Clinical Trials and Cohort Studies o Objective: support high-quality clinical trials and cohort studies that address important gaps in knowledge, leading to relevant and implementable findings for the benefit of human health o Open to research proposals for clinical trials and/or cohort studies of any size, including: › applications for new clinical trials and/or cohort studies › proposals to answer research questions using findings from existing trials or cohort studies. o Criteria: › Significance (40%) › Research Quality (40%) › Team Quality and Capability (20%) • Greater visibility and no caps • Focus on rationale (systematic review) • Design: SPIRIT and STROBE checklists • Milestone reporting

STRATEGIC AND LEVERAGING GRANTS To support research that addresses identified national needs Includes new scheme – Clinical Trials and Cohort Studies o Objective: support high-quality clinical trials and cohort studies that address important gaps in knowledge, leading to relevant and implementable findings for the benefit of human health o Open to research proposals for clinical trials and/or cohort studies of any size, including: › applications for new clinical trials and/or cohort studies › proposals to answer research questions using findings from existing trials or cohort studies. o Criteria: › Significance (40%) › Research Quality (40%) › Team Quality and Capability (20%) Applications open 6 March 2018 Minimum data due 10 April 2019 Applications close 8 May 2019 Peer review Aug/Sep 2019

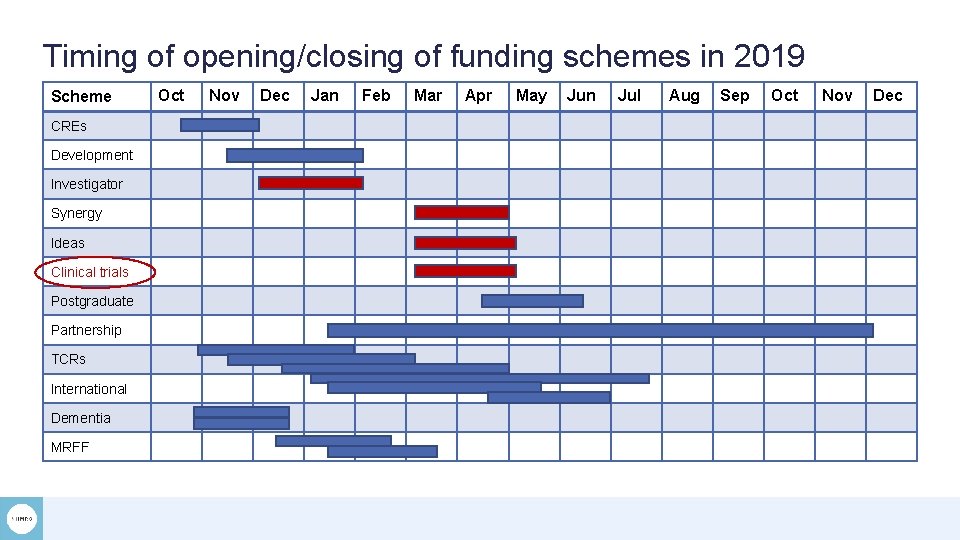
Timing of opening/closing of funding schemes in 2019 Scheme CREs Development Investigator Synergy Ideas Clinical trials Postgraduate Partnership TCRs International Dementia MRFF Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec

Medical Research Future Fund

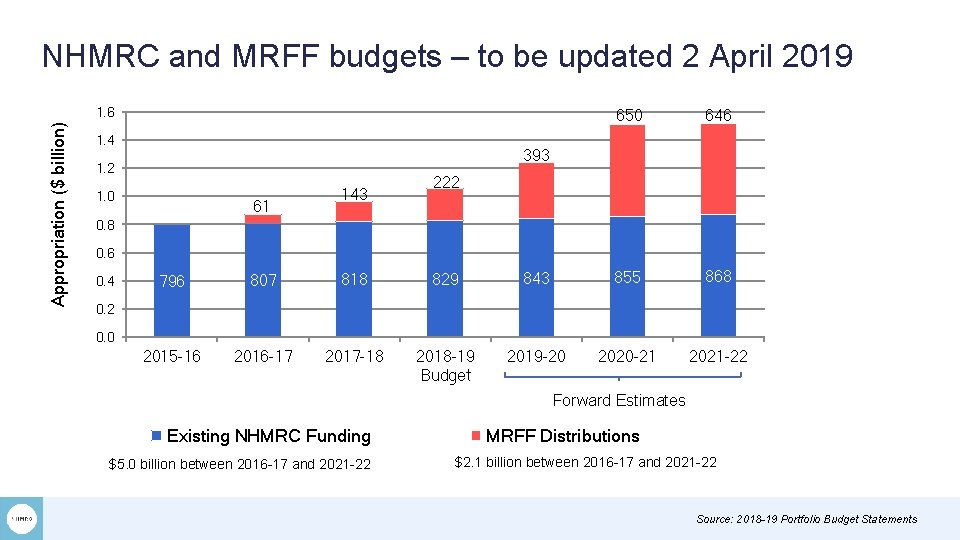
NHMRC and MRFF budgets – to be updated 2 April 2019 Appropriation ($ billion) 1. 6 1. 4 650 646 393 1. 2 1. 0 61 143 222 0. 8 0. 6 0. 4 796 807 818 829 843 855 868 2015 -16 2016 -17 2017 -18 2018 -19 Budget 2019 -20 2020 -21 2021 -22 0. 0 Forward Estimates Existing NHMRC Funding $5. 0 billion between 2016 -17 and 2021 -22 MRFF Distributions $2. 1 billion between 2016 -17 and 2021 -22 Source: 2018 -19 Portfolio Budget Statements

The future of clinical trial funding - practicalities • NHMRC will continue to support investigator-initiated clinical trials across all disease areas. • Scheme guidelines and peer review processes will need to keep up with new approaches to clinical trial design. • Scheme budgets will be reviewed annually as usual. • Scheme may evolve over time – please give us feedback. • MRFF is focussed on government priorities, which may change over time. • NHMRC will continue to deliver funding schemes (including clinical trial schemes) for MRFF at the request of Department of Health. • There are opportunities for future streamlining of NHMRC and MRFF clinical trial schemes. Watch Grant. Connect! Read the Guidelines!

The future of clinical trial funding – in 30 years? • Funders will expect: o value: clear evidence of need, potential impact and pathway to uptake o end-user engagement: participant, policy maker, healthcare provider o international linkages for recruitment and data sharing o trial registration and open data sharing o trials embedded in routine clinical practice • Funders will need to provide: o data inter-operability (with ethics applications, registries etc) o flexibility to evaluate and fund new, more efficient trial designs o more frequent/continuous funding cycles o simple reporting mechanisms

Thank you






















