2 05 Density and Specific Gravity Mass Volume





- Slides: 13

2. 05 Density and Specific Gravity Mass – Volume relationship Dr. Fred Omega Garces Chemistry 100, Miramar College 1 2. 05 Density and Specific Gravity August 2017

Eureka !!!! King Hieron wanted a new crown and so he gave the goldsmith some gold and told him to use all the gold to make a new crown. When he got his crown back it felt light. He thought that the goldsmith had cheated him and wanted Archimedes to find out if he had been duped. Archimedes did not know how to verify the authenticity of the crown initially, but he did know that if he could the density of the crown he could compare it to gold. One day, while taking a bath he got into the tub and some of the water went over the sides of the tub. He then thought of the crown. He got out of the tub and ran to the king’s palace, all naked, yelling Eureka, Eureka ( I found it, I found it). And so is the famous story of Archimedes the streaker! 2 2. 05 Density and Specific Gravity Archimedes August 2017

Density (& Specific Gravity) Physical property An observable (or measurable) property of matter i. e. , color, boiling pt, melting pt, conductivity, texture, specific heat and density. Consider what occurs when vinegar and oil is mixed. The oil floats on top and the vinegar and the herbs are at the bottom. We say that the oil is less dense than the vinegar. 3 2. 05 Density and Specific Gravity August 2017

Density : definition Density (d or ) define as: mass per unit volume. Density relates the mass to the volume for a given substance. 4 2. 05 Density and Specific Gravity August 2017

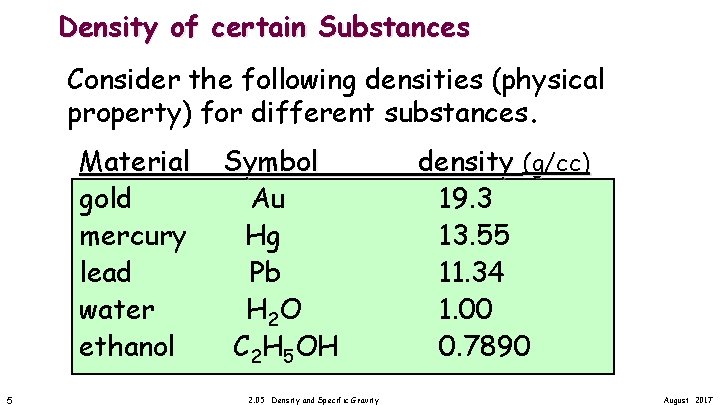
Density of certain Substances Consider the following densities (physical property) for different substances. Material gold mercury lead water ethanol 5 Symbol Au Hg Pb H 2 O C 2 H 5 OH 2. 05 Density and Specific Gravity density (g/cc) 19. 3 13. 55 11. 34 1. 00 0. 7890 August 2017

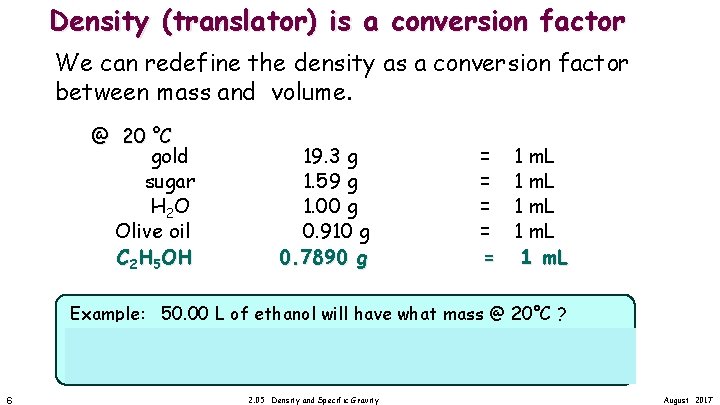
Density (translator) is a conversion factor We can redefine the density as a conversion factor between mass and volume. @ 20 °C gold sugar H 2 O Olive oil C 2 H 5 OH 19. 3 g 1. 59 g 1. 00 g 0. 910 g 0. 7890 g = = = 1 m. L Example: 50. 00 L of ethanol will have what mass @ 20°C ? = 3. 945 • 104 g 6 2. 05 Density and Specific Gravity August 2017

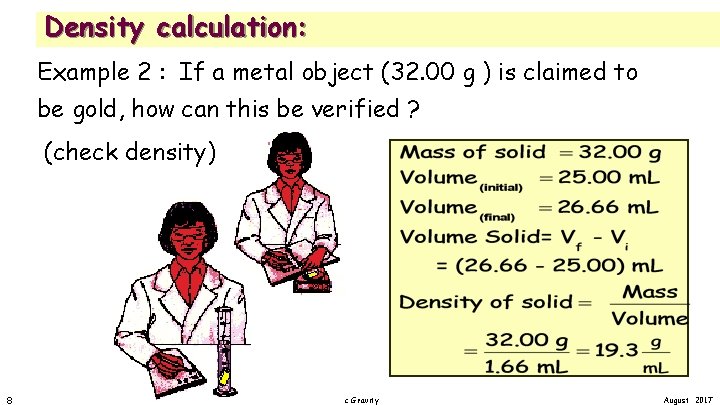
Density calculation: Example 2 : If a metal object (32. 00 g ) is claimed to be gold, how can this be verified ? (check density) 8 2. 05 Density and Specific Gravity August 2017

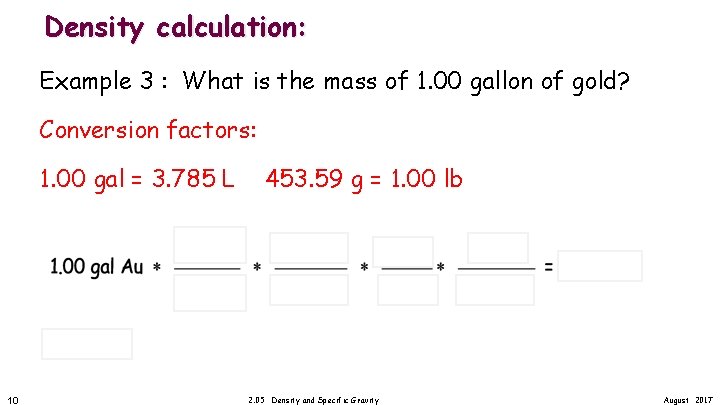
Density calculation: Example 3 : What is the mass of 1. 00 gallon of gold? In class assignment 1. 00 gal = 3. 785 L 9 2. 05 Density and Specific Gravity 453. 59 g = 1. 00 lb August 2017

Density calculation: Example 3 : What is the mass of 1. 00 gallon of gold? Conversion factors: 1. 00 gal = 3. 785 L 10 453. 59 g = 1. 00 lb 2. 05 Density and Specific Gravity August 2017

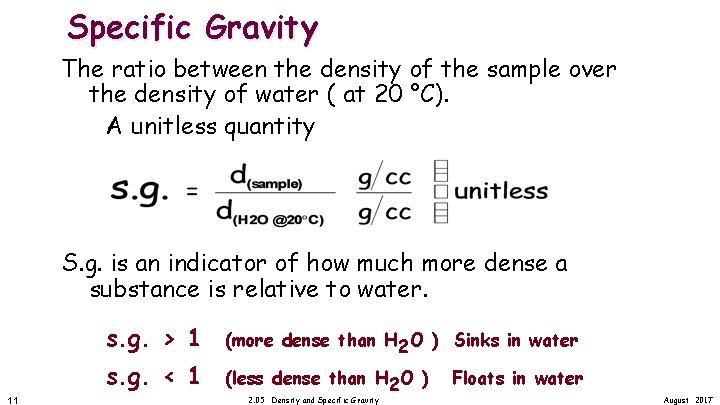
Specific Gravity The ratio between the density of the sample over the density of water ( at 20 °C). A unitless quantity S. g. is an indicator of how much more dense a substance is relative to water. s. g. > 1 s. g. < 1 11 (more dense than H 2 O ) Sinks in water (less dense than H 2 O ) 2. 05 Density and Specific Gravity Floats in water August 2017

Specific Gravity and Density In this class, we will treat Density ~ specific gravity unless otherwise stated a) A hydrometer shown measures a specific gravity over a range of 0. 700 to 0. 770. Use of hydrometers include the measurement of the specific gravity -and thus the acid content of “battery acid” in your car, the amount of alcohol The relative densities of some liquids. in wine, the sugar content of Immiscible liquids will separate into layers, with the most dense liquid going to the bottom and the least dense liquid rising to the top. 12 maple syrup and the dissolved solids in urine. B) A pycnometer is shown to hold 10 m. L. 2. 05 Density and Specific Gravity August 2017

Intensive vs Extensive Some properties of matter are quantity dependent i. e. , 50 g, 50 L If property is amount dependent call this Extensive property. Others are quantity independent i. e. , bpt H 2 O = 100°, density (H 2 O) = 1. 00 g/cc If property is amount independent call this 13 Intensive property. 2. 05 Density and Specific Gravity August 2017

Summary on Density (Specific Gravity) is a conversion factor between mass and volume. Density is an intensive property (it is amount independent). 14 2. 05 Density and Specific Gravity August 2017