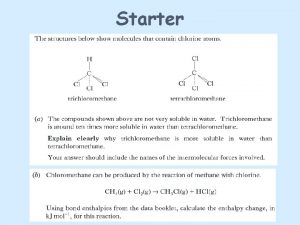
19 6 Protein Hydrolysis and Denaturation of a
- Slides: 12

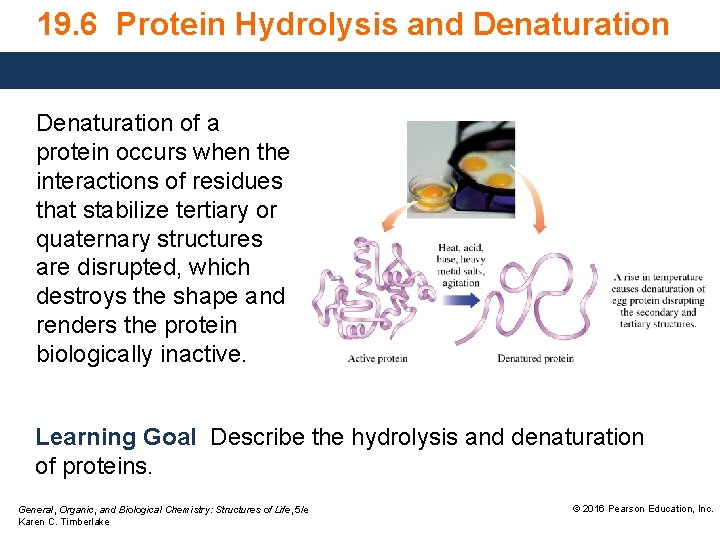
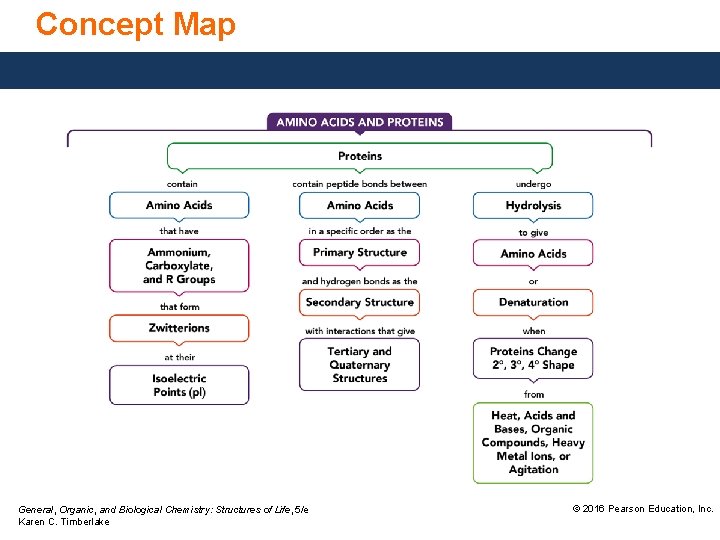
19. 6 Protein Hydrolysis and Denaturation of a protein occurs when the interactions of residues that stabilize tertiary or quaternary structures are disrupted, which destroys the shape and renders the protein biologically inactive. Learning Goal Describe the hydrolysis and denaturation of proteins. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

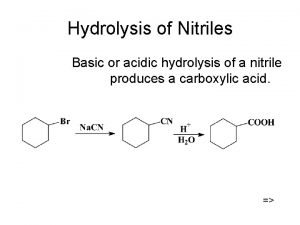
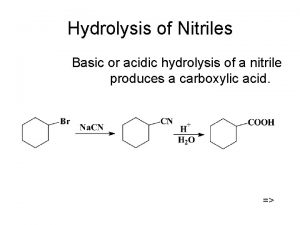
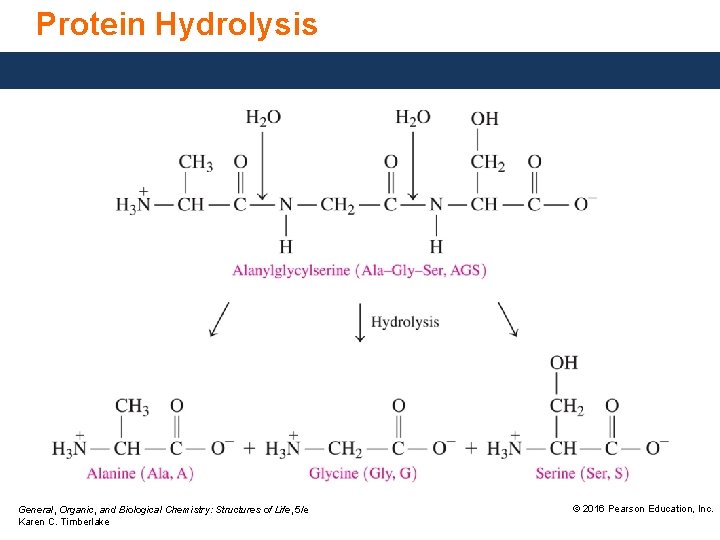
Protein Hydrolysis Peptide bonds are broken through hydrolysis reactions. Hydrolysis • occurs in the stomach when enzymes catalyze the hydrolysis of proteins to give amino acids. • breaks up the primary structure by breaking the covalent peptide bonds that link the amino acids. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Protein Hydrolysis General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

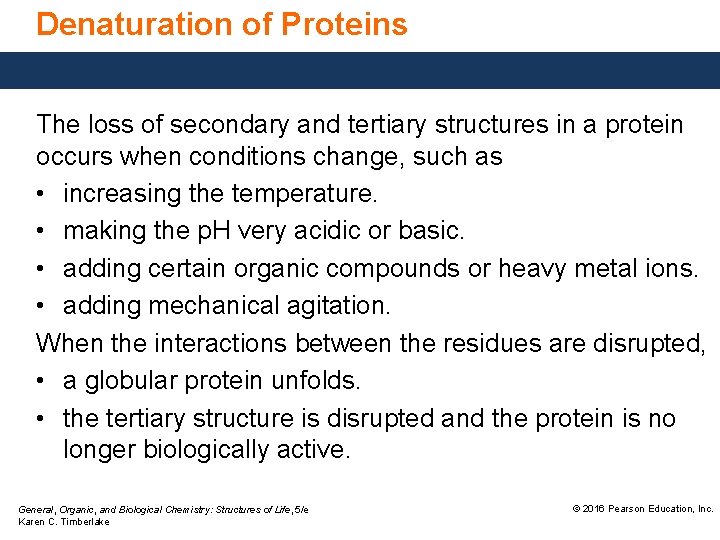
Denaturation of Proteins Denaturation of a protein • occurs when a change disrupts the interactions between residues that stabilize the secondary, tertiary, or quaternary structure. • does not affect the amide bonds between amino acids. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

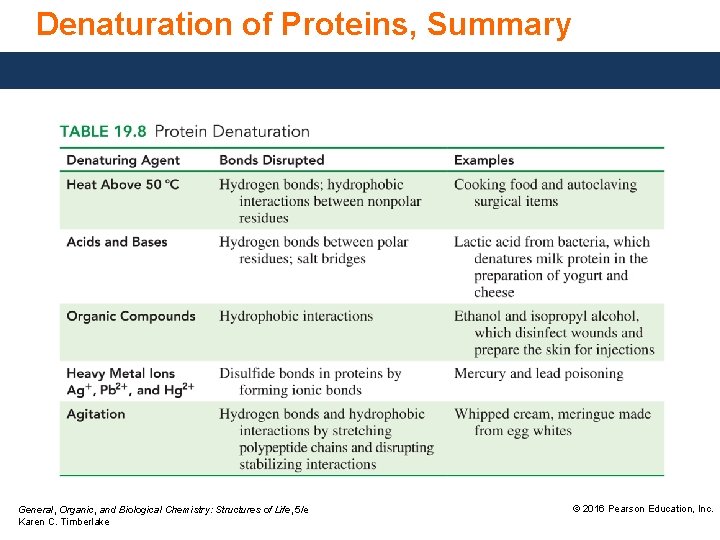
Denaturation of Proteins The loss of secondary and tertiary structures in a protein occurs when conditions change, such as • increasing the temperature. • making the p. H very acidic or basic. • adding certain organic compounds or heavy metal ions. • adding mechanical agitation. When the interactions between the residues are disrupted, • a globular protein unfolds. • the tertiary structure is disrupted and the protein is no longer biologically active. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

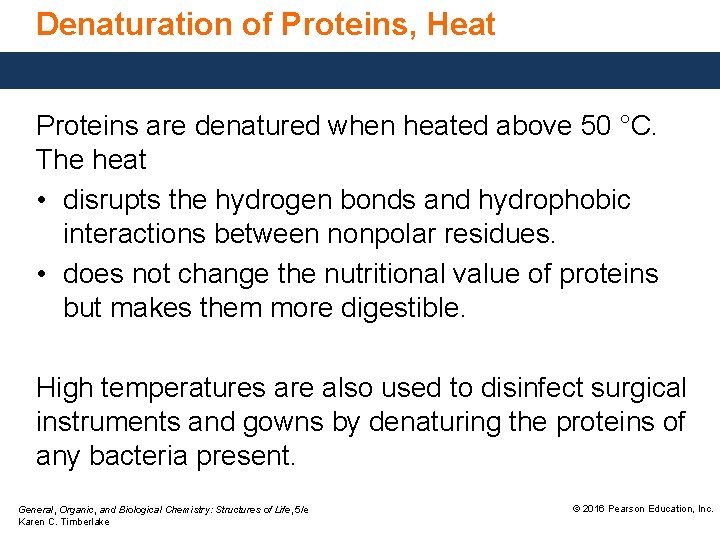
Denaturation of Proteins, Heat Proteins are denatured when heated above 50 °C. The heat • disrupts the hydrogen bonds and hydrophobic interactions between nonpolar residues. • does not change the nutritional value of proteins but makes them more digestible. High temperatures are also used to disinfect surgical instruments and gowns by denaturing the proteins of any bacteria present. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

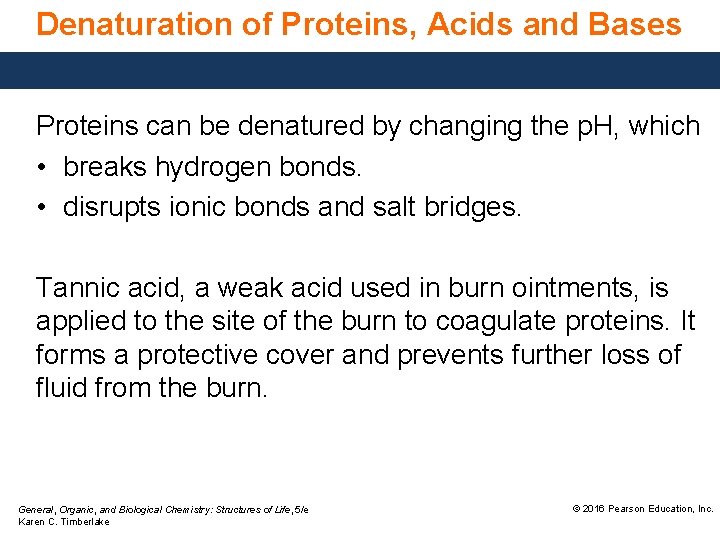
Denaturation of Proteins, Acids and Bases Proteins can be denatured by changing the p. H, which • breaks hydrogen bonds. • disrupts ionic bonds and salt bridges. Tannic acid, a weak acid used in burn ointments, is applied to the site of the burn to coagulate proteins. It forms a protective cover and prevents further loss of fluid from the burn. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

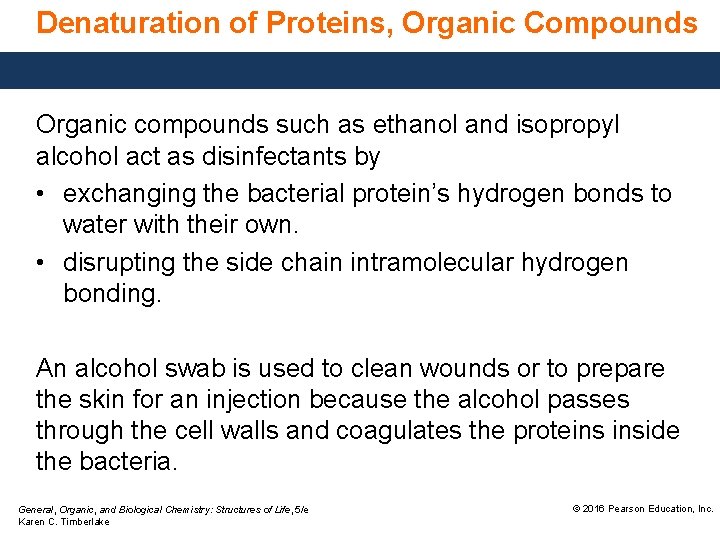
Denaturation of Proteins, Organic Compounds Organic compounds such as ethanol and isopropyl alcohol act as disinfectants by • exchanging the bacterial protein’s hydrogen bonds to water with their own. • disrupting the side chain intramolecular hydrogen bonding. An alcohol swab is used to clean wounds or to prepare the skin for an injection because the alcohol passes through the cell walls and coagulates the proteins inside the bacteria. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Denaturation of Proteins, Heavy Metal Ions Heavy metal ions such as Ag+, Pb 2+, and Hg 2+ denature proteins by forming bonds with ionic residues or reacting with disulfide — S — bonds. Dilute (1%) solutions of Ag. NO 3 are placed in the eyes of newborn babies to destroy the bacteria that cause gonorrhea. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Denaturation of Proteins, Agitation The whipping of cream and the beating of egg whites are examples of using mechanical agitation to denature proteins. The whipping action stretches the polypeptide chains until the stabilizing interactions are disrupted. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Denaturation of Proteins, Summary General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Concept Map General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
 Protein hydrolysis
Protein hydrolysis Example of denatured protein
Example of denatured protein Protein denaturation definition
Protein denaturation definition Dna denaturation definition
Dna denaturation definition Protein hydrolysis
Protein hydrolysis Thalassemia
Thalassemia Amidomalonate synthesis mechanism
Amidomalonate synthesis mechanism Protein pump vs protein channel
Protein pump vs protein channel Protein-protein docking
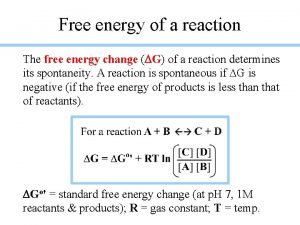
Protein-protein docking Atp hydrolysis and free energy change
Atp hydrolysis and free energy change Hydrolysis and dehydration synthesis
Hydrolysis and dehydration synthesis Dehydration synthesis and hydrolysis
Dehydration synthesis and hydrolysis Frost wedging drawing
Frost wedging drawing