17 2 17 3 Spontaneous and Nonspontaneous Processes




- Slides: 9

17. 2 -17. 3 Spontaneous and Nonspontaneous Processes - Entropy

Spontaneous vs. Nonspontaneous Processes • The AP Board now prefers the term thermodynamically favored to substitute for spontaneous although saying spontaneous will never lose points. • In thermodynamics, we must predict spontaneity. • A spontaneous process is one that does not require outside intervention. • The spontaneity of a reaction is the direction in which and extent to which a chemical reaction proceeds. (NOT HOW FAST IT OCCURS) • Predicting if a chemical process is thermodynamically favored can be challenging. In order to do so we must: – 1. ) Develop a chemical potential that predicts the direction of a chemical system. – 2. ) We must not confuse spontaneity with speed.

Chemical Reactions that are Not Thermodynamically Favored • A nonspontaneous reaction can not be made spontaneous by use of a catalyst…however, this doesn’t mean nonspontaneous reactions are impossible. Nonspontaneous reactions can be made possible by coupling them with highly spontaneous reactions. – Example: Iron can be separated from its ore if external energy , usually by means of another chemical reaction that is spontaneous, is supplied.

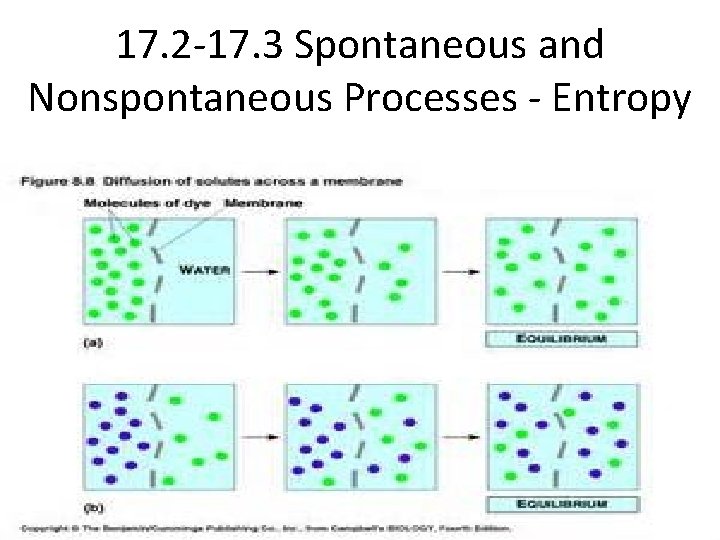
Entropy and the Second Law of Thermodynamics • Most thermodynamically favored processes are exothermic, but some are endothermic. – Examples: • Ice melting at 0 o. C • The evaporation of liquid water to gaseous water • The dissolution of sodium chloride in water – All three examples are endothermic processes and still they are spontaneous. • The reason that this is true is because the criterion for spontaneity is the entropy of the universe. • Entropy (S) – the disorder or randomness at the molecular level.

Entropy • Entropy (S) is thermodynamically function that increases with the number of energetically equivalent ways to arrange the components of a system to achieve a particular state. • S = k ln W (k is known as the Boltzmann constant (the gas constant divided by Avogadro’s number: 1. 38 x 10 -23 J/K) W is the number of energetically equivalent ways to arrange the components of a system). As W increases, so does entropy! • You will not be expected to know the equation above for the AP exam. You are however expected to understand that as the randomness of a system increases, so does entropy (S) and the value of entropy would be positive. This is because Sfinal > Sinitial, So… ΔS is positive. • For any spontaneous process, the entropy of the universe increases (ΔSuniv > 0) • Entropy, like enthalpy, is a state function…it’s value depends only on the state of the system, not on how the system arrived at that state.

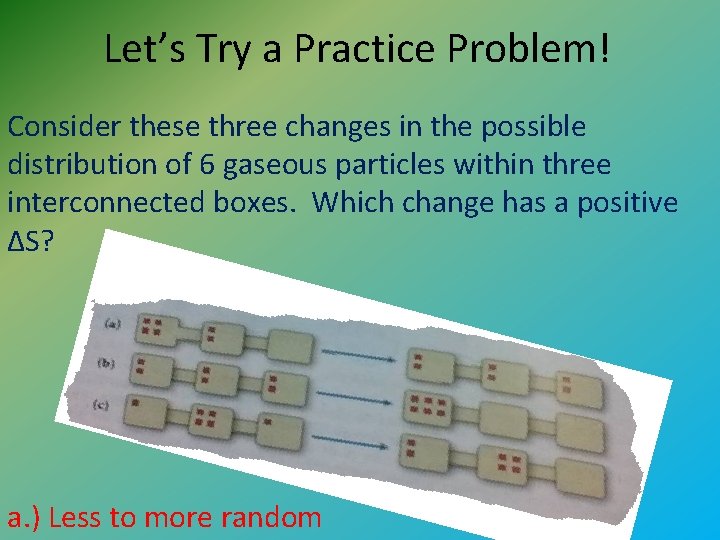
Let’s Try a Practice Problem! Consider these three changes in the possible distribution of 6 gaseous particles within three interconnected boxes. Which change has a positive ΔS? a. ) Less to more random

Entropy Changes Associated with a Change in State • Entropy increases when matter changes from a solid to a liquid, and again from a liquid to a gas. • Gases are more disordered than both solids and liquids, and a gas has more energetically equivalent configurations because it has more ways to distribute its energy. • Entropy also increases as the number of moles of gas increases during a chemical reaction.

Let’s Try Another!!! Predict the sign of ΔS for each process: a. ) the boiling of water b. ) I 2(g) I 2(s) c. ) Ca. CO 3(s) Ca. O(s) + CO 2(g) a. ) ΔS is positive b. ) ΔS is negative c. ) ΔS is positive

17. 2 -17. 3 pgs. 852 -853 #’s 28 & 32 Read 17. 4 -17. 5 pgs. 824 -831