1069 and 2827 Overview Why does this impact



















- Slides: 21

1069 and 2827 Overview

Why does this impact us all? • As goes Europe so goes the world – European regulations driving world regulations • Many examples of problems caused by trying to catch up • We need to try to make the new EU regulations as appropriate as we can – There will be knock on effects

EC 1069/2009 • Last year’s late Christmas present – Com 345/2008 • Only the articles – Very general which is good • Very different from 1774 – Nothing really to argue about – Philosophy is laudable • Implementation is critical – The devil is in the details

Strategy • Over the last few years we have built a solid working relationship with EDMA and other associations • Philosophy has been to ensure that when we can say the same thing we do • We continue to build this and are responding jointly with EDMA to 2827

Stakeholder Meeting • February 18 th, 2010 • ~40 Associations attended • Biomedical sector was represented by • ISIA • EDMA • VDGH • We couldn’t be more different!

Other Industries • • Cement Feathers Biodiesel Biogas Leather Dairy Composting Eggs (and shells)

Identification of Key Issues • We have developed a list of 11 critical concerns • European Advisory Team • VDGH • EDMA • This list is being sent to SANCO by both ISIA and EDMA • We are requesting a sector meeting to discuss these issues • What are they? ?

Key Issues • Business critical only • Purpose of the list is to drive the need for a meeting • Details can then be discussed • Proposed solutions are being identified • You can help!!!

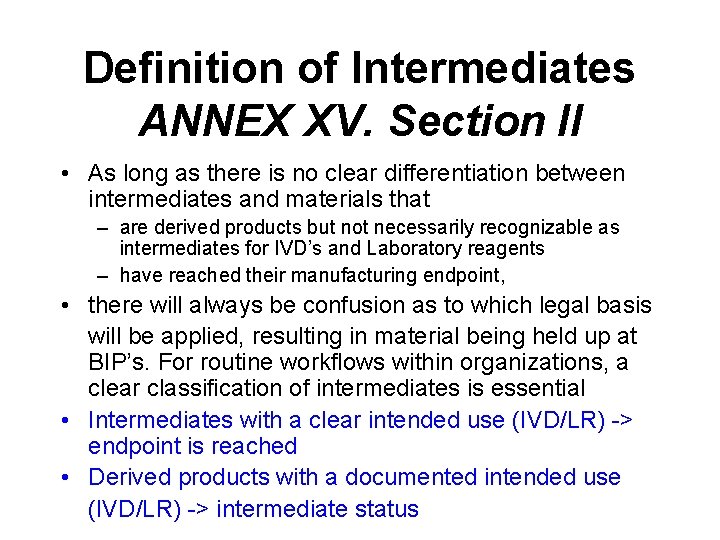
Definition of Intermediates ANNEX XV. Section II • As long as there is no clear differentiation between intermediates and materials that – are derived products but not necessarily recognizable as intermediates for IVD’s and Laboratory reagents – have reached their manufacturing endpoint, • there will always be confusion as to which legal basis will be applied, resulting in material being held up at BIP’s. For routine workflows within organizations, a clear classification of intermediates is essential • Intermediates with a clear intended use (IVD/LR) -> endpoint is reached • Derived products with a documented intended use (IVD/LR) -> intermediate status

Laboratory Reagents (Annex I, Point 28. ) • Laboratory reagents are not regulated by the EU at this time. Currently the definition only recognizes materials that are packaged and ready for the final user, but the ‘final user’ is not defined. • Non-regulated LR are linked to the biomedical R&D-sector, since LR are confined to labs. – Their use is not intended for manufacturing or therapeutic purposes. – Requirement for LR-manufacturers: registration

Endpoint of Derived Products Annex X. Section II. (Identification) • A legal basis must be developed that allows easy identification of those products which have reached an endpoint.

Third Country Registration/Approval ANNEX XV. Section II. • Interpretation of EC-requirements by 3 rd Country-Authorities and DG SANCO Directorate F are more different than intended, leading to loss of approval, approval problems and disruption of export to the EU, putting industries in the EU at a competitive disadvantage

Third Country Registration/Approval ANNEX XV. Section II. • Easy, fast registration process with renewal every > 3 years • Acceptance of registration by other agencies such as FDA • Availability of list of establishments entitled to export • Clear guidelines for all!

Categorization of Bovine Materials 96/22/EC, hormone-treatment • While Importation is possible, materials must then meet the requirements for Category 1 Material with regard to handling, treatment, approval, disposal and intra-community transport. • Handling of imported 96/22/EC-material for technical purposes should be the same as Category 3 material

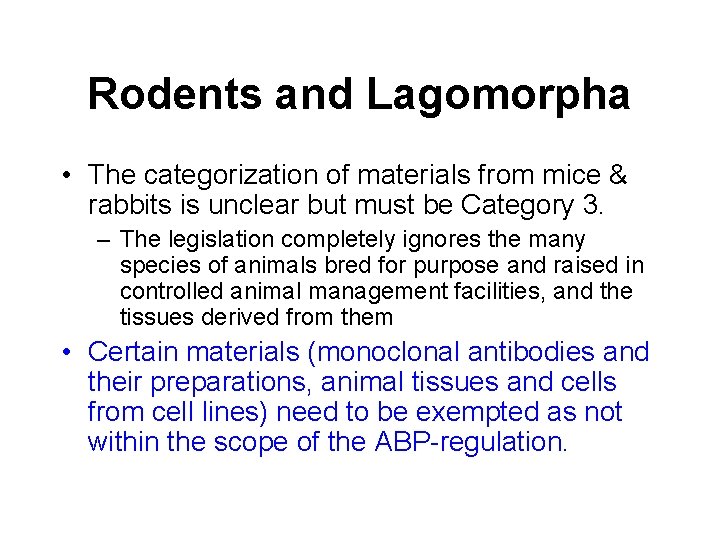
Rodents and Lagomorpha • The categorization of materials from mice & rabbits is unclear but must be Category 3. – The legislation completely ignores the many species of animals bred for purpose and raised in controlled animal management facilities, and the tissues derived from them • Certain materials (monoclonal antibodies and their preparations, animal tissues and cells from cell lines) need to be exempted as not within the scope of the ABP-regulation.

Commercial Documents Annex X. Section III • The requirement for a commercial document to accompany every intracommunity shipment will be impossible to implement as it will place a very significant administrative burden on both the recipient and the consignee. • Commercial Invoice is sufficient. – Traceability of materials is guaranteed by other means

Color-coding of Categories Annex X. Section II. (Identification) • We question the relevance of this requirement for the biomedical sector and suggest that our products be added to the list of exceptions • Exemption requested – no color-coding for any biomedical Category 3 products, derived products from rodents and lagomorphaand other laboraory animals and hormone-treated bovine materials

Storage of Category 3 material is not allowed on same site as Categories 1 & 2 ANNEX XI. Section. VI • The relevance of this for the biomedical sector is unclear and enforcement as written is impossible. • Due to the presence of large numbers of products and complex mixtures with possibly more than one category of derived products, the biomedical sector should be excluded from this requirement.

Channeling of blood or blood products (ANNEX XVII. Chapter III. • Strict channeling is required for derived blood products (Chapter 4 C). As long as the status of derived products for IVD and Laboratory Reagent use is not clarified (as being intermediates), this will remain a problem. If such channeling is required then there must be an option for reloading under specific conditions • Traceability of untreated derived blood products rather than T 5 -channeling – applicable for quantities < 1 Liter per container(? )

Bovine materials other that blood products • Meat, meat products and animal-derived materials other than blood products fall under the 96/22/EC-requirements. No legal basis exists for importing these non-blood derived products for IVD or Laboratory Reagent purposes. Examples include peptones, milk derived products and gelatin. • If used for technical purposes, these materials should be exempt,

Next Steps • Please give us any other solutions you can add • We will push for the promised sector meeting with Tim Gumbel