What makes for an effective liquid extraction The





- Slides: 8

What makes for an effective liquid extraction? The added solvent must be more volatile than the desired component. It must also specifically dissolve the desired component. The component must have a greater tendency to dissolve in the added solvent than in the solution. K D= C O = S O = CH 20 SH 20 Ratio of concentration = Ratio of solubility

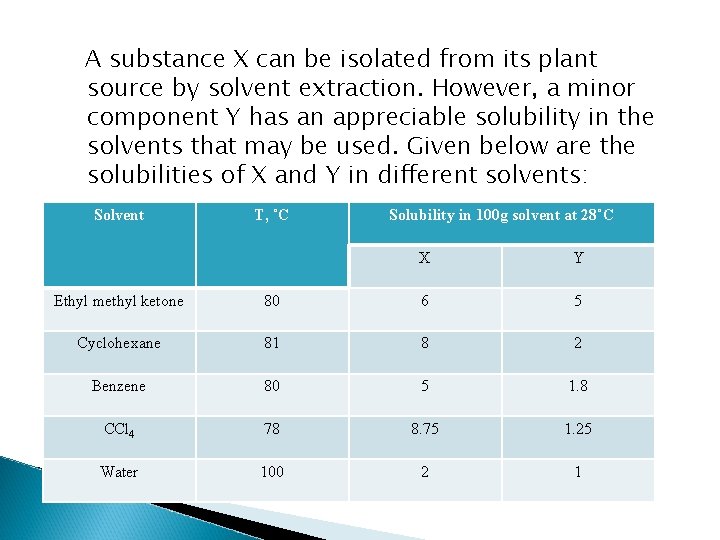
A substance X can be isolated from its plant source by solvent extraction. However, a minor component Y has an appreciable solubility in the solvents that may be used. Given below are the solubilities of X and Y in different solvents: Solvent T, ˚C Solubility in 100 g solvent at 28˚C X Y Ethyl methyl ketone 80 6 5 Cyclohexane 81 8 2 Benzene 80 5 1. 8 CCl 4 78 8. 75 1. 25 Water 100 2 1

Answers to Questions a. Which is the best extracting solvent? CCl 4 is the best extracting solvent it dissolves only a little amount of Y relative to the X component needed. It also dissolves the greatest amount of X among the choices. It is immiscible with water, which happens to be the solvent in the solution.

Answers to Questions ◦ Mathematically speaking, the choice relies on the KD and the best result is the one with the highest KD. The KD values with respect to water as calculated are the following: Solvent KD Ethyl methyl ketone 3 Cyclohexane 4 Benzene 2. 5 CCl 4 4. 375 ◦ From this table, it is evident that CCl 4 is the yields the highest ratio, ergo the best possible choice as the extracting solvent.

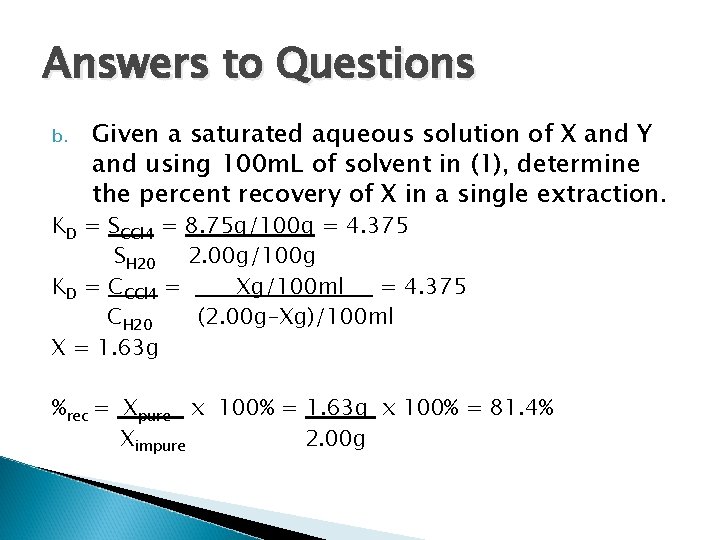
Answers to Questions b. Given a saturated aqueous solution of X and Y and using 100 m. L of solvent in (1), determine the percent recovery of X in a single extraction. KD = SCCl 4 = 8. 75 g/100 g = 4. 375 SH 20 2. 00 g/100 g KD = CCCl 4 = Xg/100 ml = 4. 375 CH 20 (2. 00 g-Xg)/100 ml X = 1. 63 g %rec = Xpure x 100% = 1. 63 g x 100% = 81. 4% Ximpure 2. 00 g

Answers to Questions c. Repeat (b) using 50 m. L of solvent in each of the two successive extractions. Determine the percent recovery and compare this with (b). KD = SCCl 4 = 8. 75 g/100 g = 4. 375 SH 20 2. 00 g/100 g First extraction: KD = CCCl 4 = Xg/50 ml = 4. 375 CH 20 (2. 00 g-Xg)/100 ml X = 1. 37 g

Answers to Questions Second extraction: KD = CCCl 4 = Xg/50 ml = 4. 375 CH 20 (0. 63 g-Xg)/100 ml X = 0. 43 g %rec = Xpure x 100%= (1. 37+0. 43)g x 100%= 90. 0% Ximpure 2. 00 g In the double extraction, the percent recovery is comparably higher, considering the same amount of the solvent added.

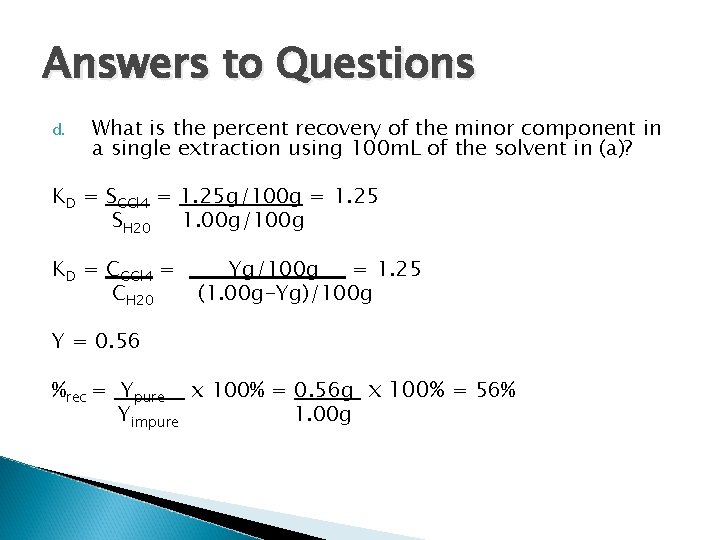
Answers to Questions d. What is the percent recovery of the minor component in a single extraction using 100 m. L of the solvent in (a)? KD = SCCl 4 = 1. 25 g/100 g = 1. 25 SH 20 1. 00 g/100 g KD = CCCl 4 = CH 20 Y = 0. 56 Yg/100 g = 1. 25 (1. 00 g-Yg)/100 g %rec = Ypure x 100% = 0. 56 g x 100% = 56% Yimpure 1. 00 g