What is Early Access Program Early Access Programs









- Slides: 10



What is Early Access Program? Early Access Programs provide access to medicines outside the commercial or clinical trial setting (Pre- & Peri- Approval access) in the following situations: 1. Access to unapproved product during the development phase 2. Α. Access to product σημείωμα approved by major regulatory authority but not Εισαγωγικό in country of request * (e. g. USA) 3. Post-trial access (continued access)

Early Access Programs Lorem ipsum dolor sit amet, consectetur adipiscing elit, consectetur adipiscing Greek legislation: FEK 558/8 -4 -11 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. • Terms, conditions and procedure for granting temporary Lorem ipsum dolor sitto amet, consectetur adipiscing elit, sed for do eiusmod temporuse incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, early access medicinal products human quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. ("palliative use") Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. • 1. “Early access" to medicinal products for human use ("palliative use") means the provision, for humanitarian reasons, adipiscing to a group suffering a chronic Lorem ipsum dolor sit amet, consectetur elit, of sedpatients do eiusmod temporfrom incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, or severe disability or life-threatening risk for which satisfactory treatment with quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum authorized medicinal products has not achieved, medicinal falling dolore eu fugiat nulla pariatur. Excepteur sint been occaecat cupidatatanon proident, product sunt in culpa qui officia deserunt mollit anim id est laborum. either in categories (specific legislation) and which is either the subject of an application for a marketing authorization in accordance with the procedures of the abovementioned Regulation (specific legislation) or is in the clinical trial stage, clinical trial data leading in principle to positive results, as defined in the Joint Ministerial Decision (specific legislation) on clinical trials of medicinal products for human use. • Early access program is not a clinical study.

EAP Philosophy & Principles • EAPs encompass a broad set of terminology* : • CUP Compassionate Use Program, • EAP Early or Expanded Access Program, • NPS Named Patient Supply, MPP Multiple Patient Program, • IPP Individual Patient Program, • ETP Expanded Treatment Protocol etc. Business Use Only 5

Lorem ipsum dolor sit amet, consectetur adipiscing elit, consectetur adipiscing Criteria for all EAPs Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, exercitation ullamcoshould laboris nisi aliquip exfrom ea commodo consequat. Duis auteorirure dolor. Authorities in reprehenderit voluptate velit esse cillum • quis Annostrud independent request be ut received the treating physician, Health or in Institutions dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. • The patient to be treated has a serious or life threatening disease or condition, and no comparable or satisfactory Lorem ipsum dolor sit amet, adipiscing elit, or sed do eiusmod temporor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, alternative therapy isconsectetur available to monitor treat the disease condition quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum • dolore Theeu patient is not eligible to enroll a clinical trial non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. fugiat nulla pariatur. Excepteur sintin occaecat cupidatat • Lorem There is adolor potential patient benefit to justify thedopotential risk ofincididunt the treatment andmagna the potential risk isadnot ipsum sit amet, consectetur adipiscing elit, sed eiusmod tempor ut labore use, et dolore aliqua. Ut enim minim veniam, unreasonable in theullamco context of the disease orea condition be treated quis nostrud exercitation laboris nisi ut aliquip ex commodoto consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. • Provision of the investigational product will not interfere with the initiation, conduct or completion of a clinical trial or Lorem ipsum dolor sit amet, program consecteturand adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, overall development nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum • quis Sucheuaccess provision described allowednon as proident, per localsunt laws and regulations dolore fugiat nulla pariatur. as. Excepteur sint above occaecatiscupidatat in culpa qui officia deserunt mollit anim id est laborum. *The above terms may have a specific regulatory / legal framework at a local level, which may vary across and from US/EU legislation that is evolving

Lorem ipsum dolor sit amet, consectetur adipiscing elit, consectetur adipiscing Greek Legislation Requirements ü * according to EOF FEK 558 / 8 -4 -11 ". . . if it is a medicine for which clinical trials are being conducted, they should be at the Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, finalquis stage andexercitation have preliminary results patients for whom it is irure intended. that case, in a voluptate relevantvelit statement from nostrud ullamco laboris nisifavorable ut aliquip exto eathe commodo consequat. Duis aute dolor in. In reprehenderit esse cillum fugiatresponsible nulla pariatur. will Excepteur sint occaecat proident, sunt infor culpa qui officia deserunt mollit anim id est laborum. the dolore early eu access be made that hecupidatat / she isnon bound to apply a marketing authorization within the immediately following time dolor period and, consectetur however, adipiscing not moreelit, than application foretprovisional of minim early veniam, access to Lorem ipsum sit amet, sed 7 domonths eiusmod from temporthe incididunt ut labore dolore magnaauthorization aliqua. Ut enim ad the quis medicinal product. . . ullamco Any positive evaluation European Medicines orinthe Competent Authority ofesse another nostrud exercitation laboris nisi ut aliquip exby ea the commodo consequat. Duis aute. Agency irure dolor reprehenderit in voluptate velit cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. Member State and Analytical Information pursuant to Article 83 (2) of Regulation 726/2004 / EC and the relevant Community guidelines. Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrudstatement exercitation by ullamco laboris nisi ut aliquip ex ea consequat. auteisirure dolor in reprehenderit in voluptate velit ü Responsible the person responsible forcommodo early access that. Duis there no rejection of his or her request oresse anycillum relevant dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. negative opinion from the European Medicines Agency or the CHMP (Committee for Medicinal Products for Human Use) or another Member Statedolor. . . sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, Lorem ipsum quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. EMA approval date is effective for local Greek approval *according to local Greek legislation (EOF FEK 558/8 -4 -11) "Once the medicine is approved, the temporary early access authorization shall cease to be valid and the Early Access Responsible shall immediately inform the treating physician and the participating patients to whom the medicinal product is granted under the terms of the marketing authorization. "

Lorem ipsum dolor sit amet, consectetur adipiscing elit, consectetur adipiscing Greek Legislation Requirements Prior to launching the group program, the "Early Product Access Program Team" shall submit to the EOF. . . Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, • nostrud. . f)exercitation The responsible medical the respective specialty that they exhausted or with velit esse cillum quis ullamco laboris nisistatement ut aliquip ex of ea commodo consequat. Duis aute irure dolorare in reprehenderit in voluptate certainty will exhaust the existing therapies. . non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat • ipsum The "Early Responcible shallelit, promptly notify tempor to theincididunt EOF the ut specific Public physicians Lorem dolor sit. Access" amet, consectetur adipiscing sed do eiusmod labore et dolore. Hospitals, magna aliqua. Ut enim ad minim veniam, and, exercitation quarterly, ullamco the patients who joinedexthe approvedconsequat. "Early Access Program" quis nostrud laboris nisi ut aliquip ea commodo Duis aute irure dolor. . . in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. The medicine that is the subject of a temporary early access license is handled under the responsibility of the hospital pharmacist. Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis exercitation ullamco laboris nisi ut aliquip exafter ea commodo consequat. aute program irure dolor for in reprehenderit in refers voluptate • nostrud Labeling of medicinal products granted the adoption of a. Duis group early access to: velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. • • (a) the name of the medicinal product or where the code name and the active ingredient (s) are present. Lorem ipsum consectetur adipiscing elit, sed doheadquarters, eiusmod tempor incididuntnumber ut laboreand et dolore magna aliqua. enim ad minim veniam, • (b)dolor namesitoramet, business name, where appropriate, telephone other details of the Ut person quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum responsible for the Excepteur program sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. dolore eu fugiat nulla pariatur. • • • (c) the pharmaceutical form and the route of administration (d) the batch number and the expiry date (e) storage conditions (f) instructions for use (refer to leaflet or other explanatory document) (g) the phrase "Early access medicine - not sold" EOF publishes on its website a list of the medicines that are the subject of an approved group early access program.

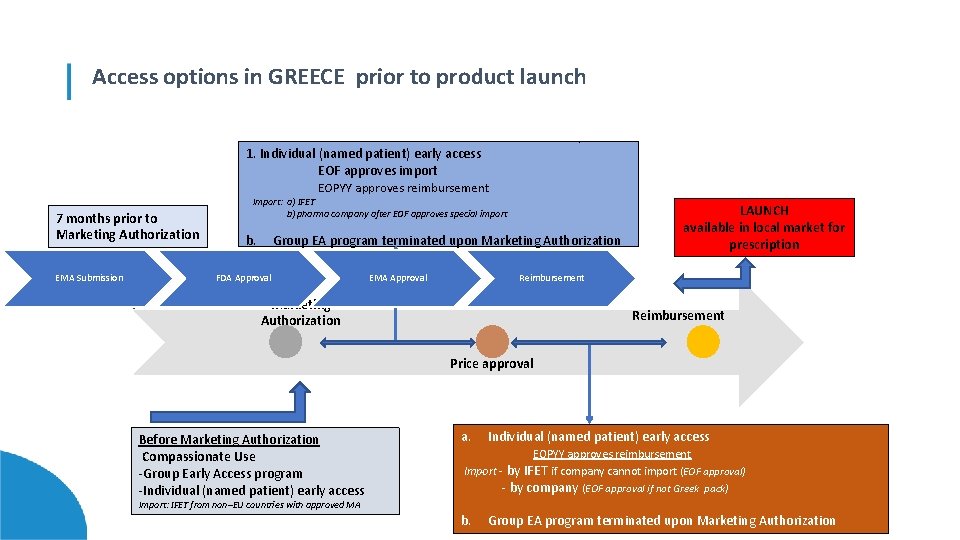
Lorem ipsum dolor in sit GREECE amet, consectetur adipiscing elit, launch consectetur adipiscing Access options prior to product Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute. irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. occaecat cupidatat proident, sunt in culpa qui officia deserunt mollit anim id est laborum. 1. Excepteur Individualsint (named patient) earlynon access EOF approves import Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, EOPYY approves quis nostrud exercitation ullamco laboris nisi ut aliquip ex eareimbursement commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum Import: a) IFET dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. LAUNCH b) pharma company after EOF approves special import 7 months prior to available in local market for Marketing Authorization Lorem ipsum dolor sit amet, consectetur elit, terminated sed do eiusmod incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, b. Group adipiscing EA program upontempor Marketing Authorization quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderitprescription in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. EMA Submission FDA Approval EMA Approval Reimbursement Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco Marketing laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia. Reimbursement deserunt mollit anim id est laborum. Authorization Price approval Before Marketing Authorization Compassionate Use -Group Early Access program -Individual (named patient) early access a. Individual (named patient) early access EOPYY approves reimbursement Import - by IFET if company cannot import (EOF approval) - by company (EOF approval if not Greek pack) Import: IFET from non–EU countries with approved MA b. Group EA program terminated upon Marketing Authorization

Thank you Any Questions?