Welcome to Year 10 Chemistry Mrs Tonge Leaving









- Slides: 15

Welcome to Year 10 Chemistry Mrs Tonge

Leaving a blank page for your Title Page… • Write the title, “Quick Quiz on Year 9 Chemistry” • Write out the numbers 1 -10, leaving a line between each number….

Quick Quiz 1. What are three states of matter? 2. Name 2 scientific words used to describe the properties of a metal. 3. Define the term ‘solvent’. 4. What is the difference between an element and a compound?

5. Explain ‘condensation’ 6. What would you do to separate a mixture of iron and sand? 7. What would you do to separate a mixture of salt and sand? 8. What does water do when it reaches 100 OC?

9. What are three main components of an atom? 10. What is the chemical symbol for: a) OXYGEN b) SODIUM Lets see how you have done, I will be recording the marks.

Atomic Model Changes through the Ages. .

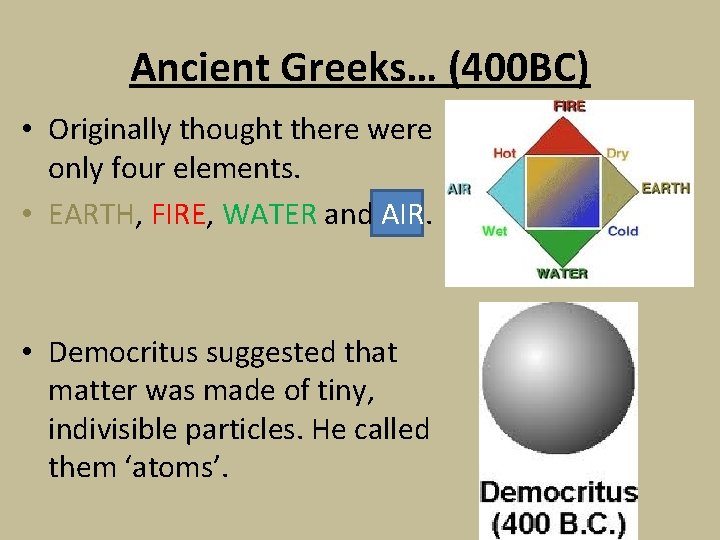
Ancient Greeks… (400 BC) • Originally thought there were only four elements. • EARTH, FIRE, WATER and AIR. • Democritus suggested that matter was made of tiny, indivisible particles. He called them ‘atoms’.

JOHN DALTON, 1808 • Stated matter is made of indivisible atoms, like a billiard ball. • Atoms cannot be made or destroyed, possessing different masses and properties.

JJ THOMPSON, 1898 • Noted that atoms have positive and negative parts. • Thought atoms were positive, with negative parts scattered throughout. • Name the “plum pudding” model

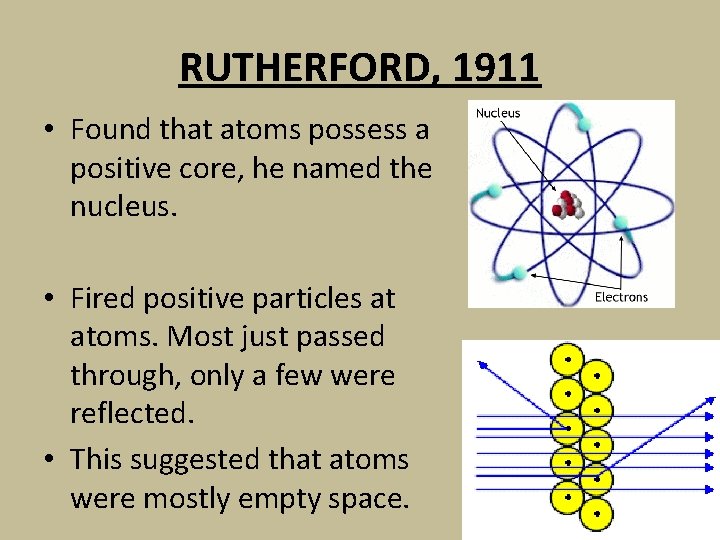
RUTHERFORD, 1911 • Found that atoms possess a positive core, he named the nucleus. • Fired positive particles at atoms. Most just passed through, only a few were reflected. • This suggested that atoms were mostly empty space.

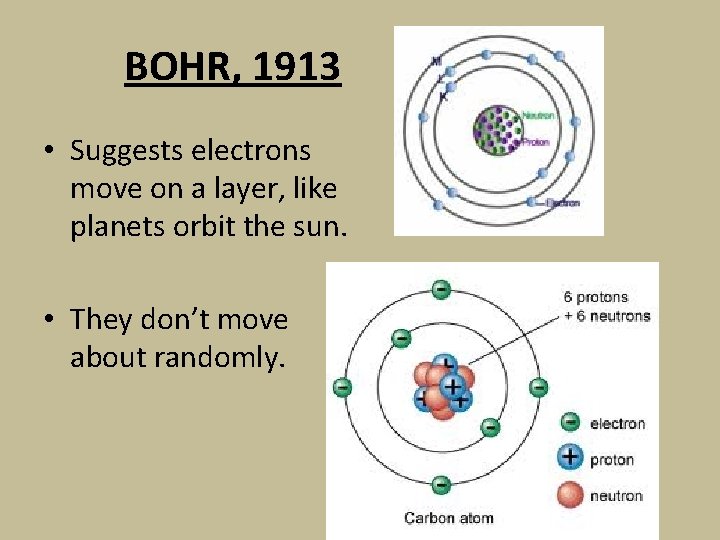
BOHR, 1913 • Suggests electrons move on a layer, like planets orbit the sun. • They don’t move about randomly.

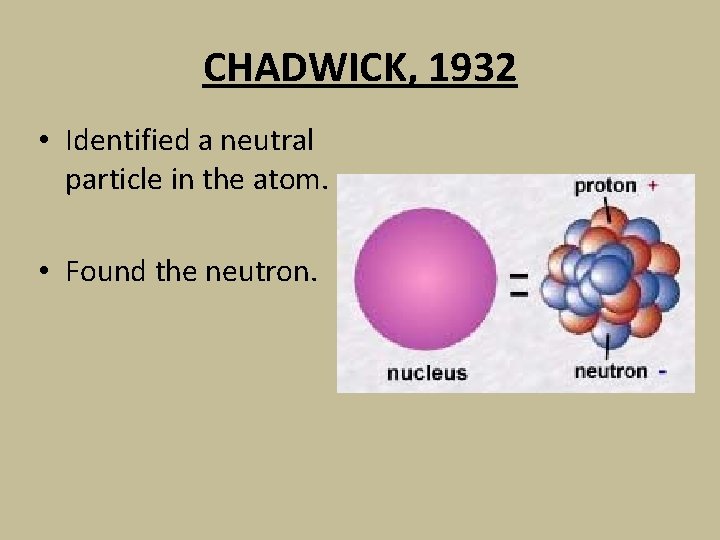
CHADWICK, 1932 • Identified a neutral particle in the atom. • Found the neutron.

QUANTUM MECHANICS, “today” • Electrons NOT on shells, but located moving within clouds.


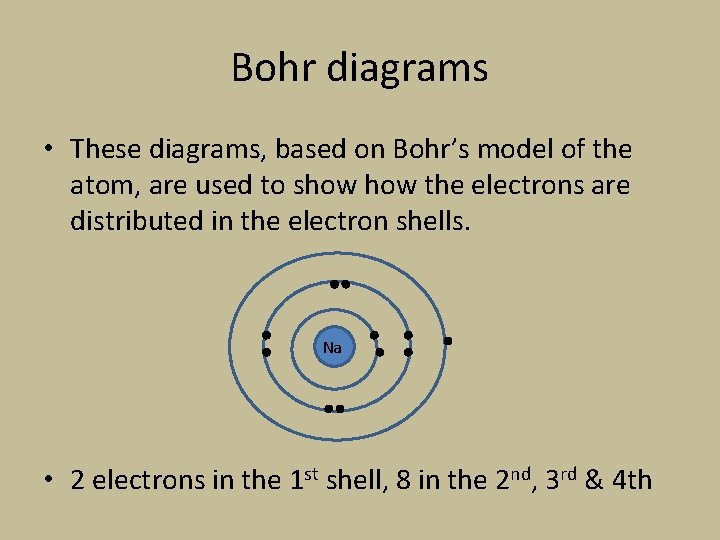
Bohr diagrams • These diagrams, based on Bohr’s model of the atom, are used to show the electrons are distributed in the electron shells. Na • 2 electrons in the 1 st shell, 8 in the 2 nd, 3 rd & 4 th
 Leaver poem
Leaver poem Talle tonge
Talle tonge Talle tonge mathews phosa
Talle tonge mathews phosa Talle tonge mathews phosa
Talle tonge mathews phosa Leaving group
Leaving group They are mrs garcia and mrs castro
They are mrs garcia and mrs castro They are mrs garcia and mrs castro
They are mrs garcia and mrs castro Mrs. darling was ___________ of mrs. s.
Mrs. darling was ___________ of mrs. s. Mrs jones computer lab
Mrs jones computer lab Welcome to year 11
Welcome to year 11 Welcome to year 8
Welcome to year 8 Welcome to your senior year of high school
Welcome to your senior year of high school Welcome to year 5
Welcome to year 5 Attendance90rule
Attendance90rule Welcome welcome this is our christmas story
Welcome welcome this is our christmas story Welcome to chemistry class
Welcome to chemistry class


























