Warm UP 1111 1112 Complete the following specific








- Slides: 11

Warm- UP 11/11 & 11/12 Complete the following specific heat problem: What mass of water is needed to raise the heat of water by 141, 277. 5 joules as the temperature changes by 45°C?

Tues. 11/11 & Wed. 11/12 Objective (Learning Target) - I can identify and analyze different types of reactions by participating in a cooperative learning card sort. & I can compose balanced chemical reactions by completing a worksheet successfully. Agenda: 1. Answer questions on Specific Heat HW. 2. Chemical Reactions 3. Balancing Chemical Equations HW: Balancing equations worksheet

Quote of Inspiration “A thinker sees his own actions as experiments and questions--as attempts to find out something. Success and failure are for him answers above all. ” ― Friedrich Nietzsche

Brainstorm Show me what you’re working with! Lets “popcorn” (brainstorm) some things we know about chemical reactions!

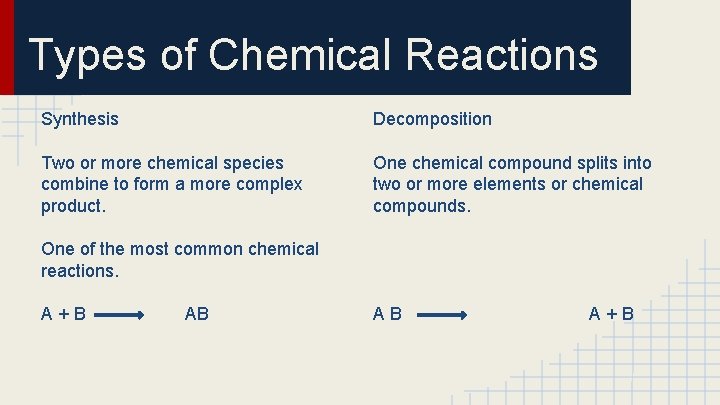
Types of Chemical Reactions Synthesis Decomposition Two or more chemical species combine to form a more complex product. One chemical compound splits into two or more elements or chemical compounds. One of the most common chemical reactions. A+B AB AB A+B

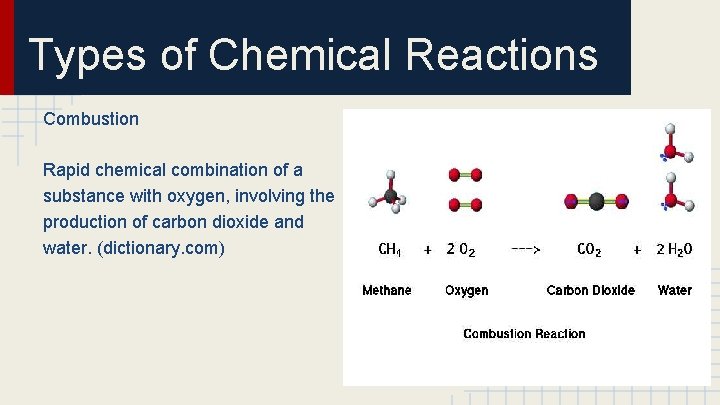
Types of Chemical Reactions Combustion Rapid chemical combination of a substance with oxygen, involving the production of carbon dioxide and water. (dictionary. com)

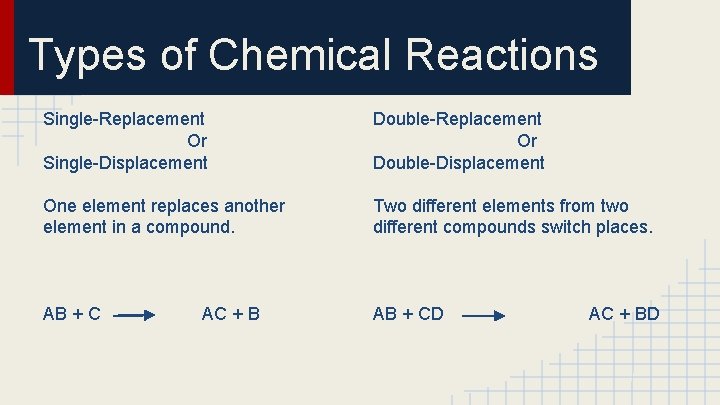
Types of Chemical Reactions Single-Replacement Or Single-Displacement Double-Replacement Or Double-Displacement One element replaces another element in a compound. Two different elements from two different compounds switch places. AB + CD AC + BD

Balancing Equations I do, We do, You do… I will do the first example, We will do the last two, You will complete an activity!

“Building an Army” ● ● ● I will put you into teams of 2 You will be given a form to complete throughout the activity. Your team will pick a snowman and turn it over. A chemical equation will be written on the back. Your group will balance the equation and write in on your form along with the number from your snowman. ● Think you have it correct? Dr. Pitot and I will be check them as your group raises your hands. ● Get it right, you get to move onto the next snowman (entire group must get it right!). ● Winner will complete the most snowman and have the largest army!

Lab Write Up Look over the comments and feedback given on your lab write ups. Using your rubric (success criteria): ● Self assess o What did I do correct? What do I need to work on? ● Questions? Concerns? Comments? Come see me PLEASE!

Cool-Down What type of reaction is shown below? Balance the equation Mg + Na. OH STAY WARM! Mg(OH)2 + Na Success Criteria - I can identify and analyze different types of reactions & I can successfully balance chemical equations