Thermal Physics Lecture 5 Dr Ing Erwin Sitompul



- Slides: 16

Thermal Physics Lecture 5 Dr. -Ing. Erwin Sitompul President University http: //zitompul. wordpress. com 2 0 1 4 President University Erwin Sitompul Thermal Physics 5/1

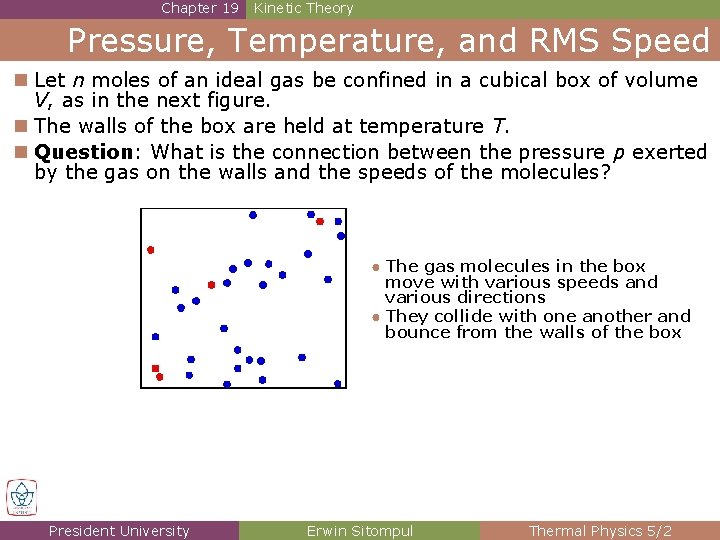
Chapter 19 Kinetic Theory Pressure, Temperature, and RMS Speed n Let n moles of an ideal gas be confined in a cubical box of volume V, as in the next figure. n The walls of the box are held at temperature T. n Question: What is the connection between the pressure p exerted by the gas on the walls and the speeds of the molecules? ● The gas molecules in the box move with various speeds and various directions ● They collide with one another and bounce from the walls of the box President University Erwin Sitompul Thermal Physics 5/2

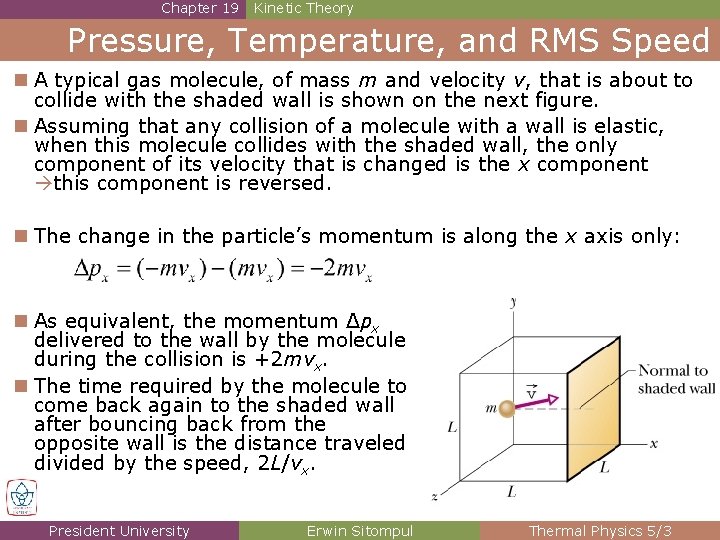
Chapter 19 Kinetic Theory Pressure, Temperature, and RMS Speed n A typical gas molecule, of mass m and velocity v, that is about to collide with the shaded wall is shown on the next figure. n Assuming that any collision of a molecule with a wall is elastic, when this molecule collides with the shaded wall, the only component of its velocity that is changed is the x component this component is reversed. n The change in the particle’s momentum is along the x axis only: n As equivalent, the momentum Δpx delivered to the wall by the molecule during the collision is +2 mvx. n The time required by the molecule to come back again to the shaded wall after bouncing back from the opposite wall is the distance traveled divided by the speed, 2 L/vx. President University Erwin Sitompul Thermal Physics 5/3

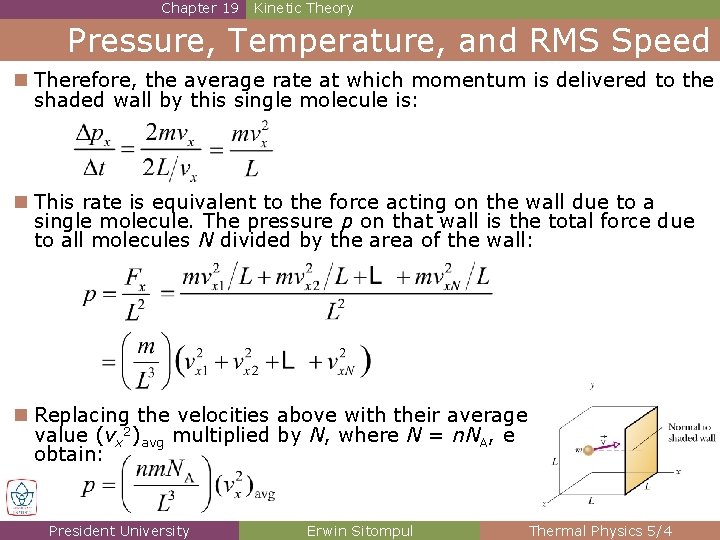
Chapter 19 Kinetic Theory Pressure, Temperature, and RMS Speed n Therefore, the average rate at which momentum is delivered to the shaded wall by this single molecule is: n This rate is equivalent to the force acting on the wall due to a single molecule. The pressure p on that wall is the total force due to all molecules N divided by the area of the wall: n Replacing the velocities above with their average value (vx 2)avg multiplied by N, where N = n. NA, e obtain: President University Erwin Sitompul Thermal Physics 5/4

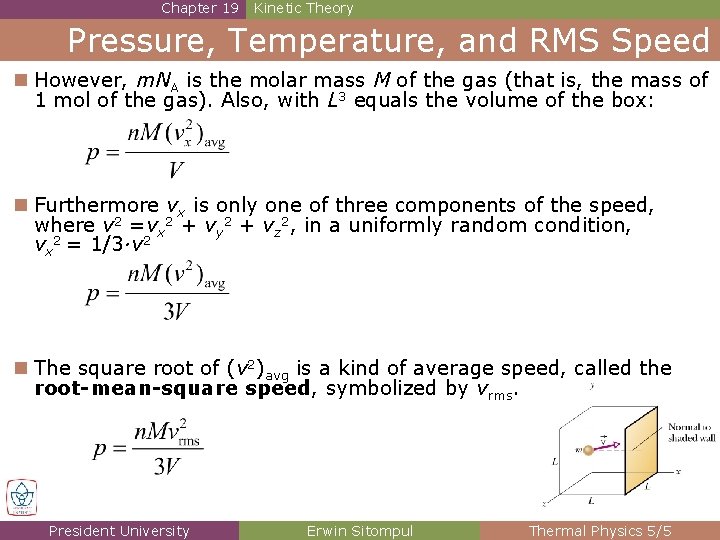
Chapter 19 Kinetic Theory Pressure, Temperature, and RMS Speed n However, m. NA is the molar mass M of the gas (that is, the mass of 1 mol of the gas). Also, with L 3 equals the volume of the box: n Furthermore vx is only one of three components of the speed, where v 2 =vx 2 + vy 2 + vz 2, in a uniformly random condition, vx 2 = 1/3·v 2 n The square root of (v 2)avg is a kind of average speed, called the root-mean-square speed, symbolized by vrms. President University Erwin Sitompul Thermal Physics 5/5

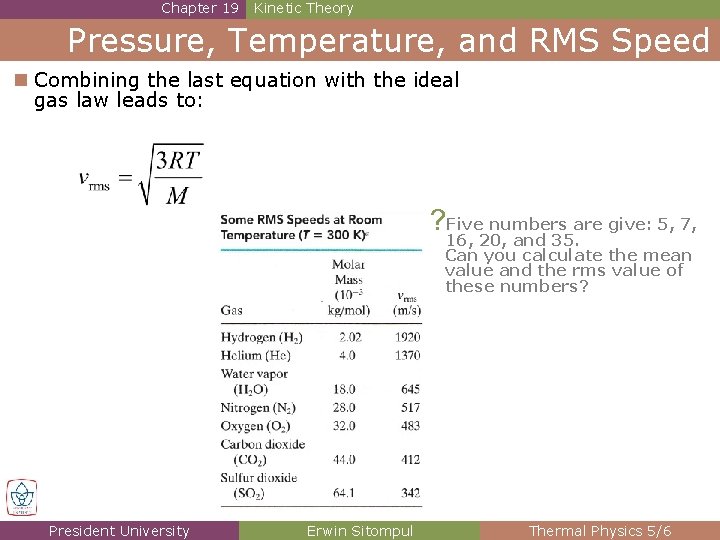
Chapter 19 Kinetic Theory Pressure, Temperature, and RMS Speed n Combining the last equation with the ideal gas law leads to: ? Five numbers are give: 5, 7, 16, 20, and 35. Can you calculate the mean value and the rms value of these numbers? President University Erwin Sitompul Thermal Physics 5/6

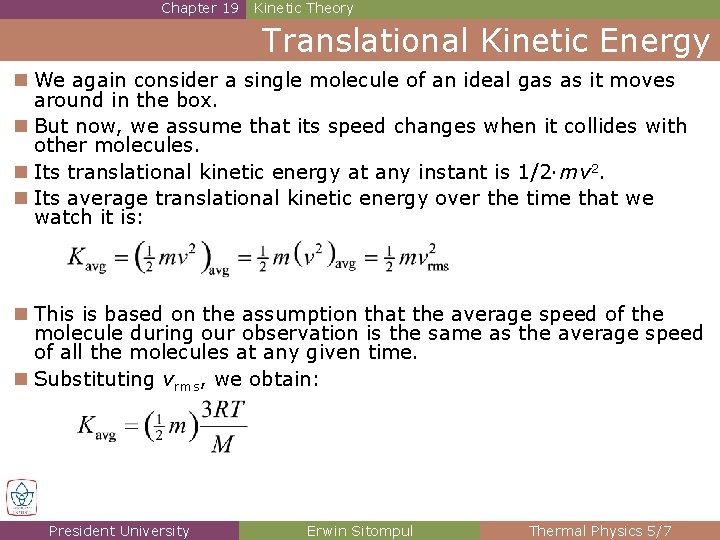
Chapter 19 Kinetic Theory Translational Kinetic Energy n We again consider a single molecule of an ideal gas as it moves around in the box. n But now, we assume that its speed changes when it collides with other molecules. n Its translational kinetic energy at any instant is 1/2·mv 2. n Its average translational kinetic energy over the time that we watch it is: n This is based on the assumption that the average speed of the molecule during our observation is the same as the average speed of all the molecules at any given time. n Substituting vrms, we obtain: President University Erwin Sitompul Thermal Physics 5/7

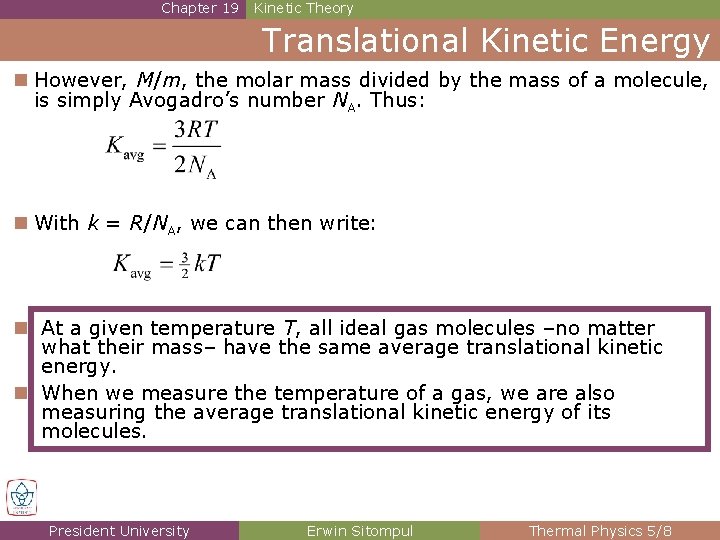
Chapter 19 Kinetic Theory Translational Kinetic Energy n However, M/m, the molar mass divided by the mass of a molecule, is simply Avogadro’s number NA. Thus: n With k = R/NA, we can then write: n At a given temperature T, all ideal gas molecules –no matter what their mass– have the same average translational kinetic energy. n When we measure the temperature of a gas, we are also measuring the average translational kinetic energy of its molecules. President University Erwin Sitompul Thermal Physics 5/8

Chapter 19 Kinetic Theory Checkpoint A gas mixture consists of molecules of types 1, 2, and 3, with molecular masses m 1 > m 2 > m 3. Rank the three types according to (a) average kinetic energy and (b) rms speed, greatest first. (a) All tie. (b) 3, 2, then 1 President University Erwin Sitompul Thermal Physics 5/9

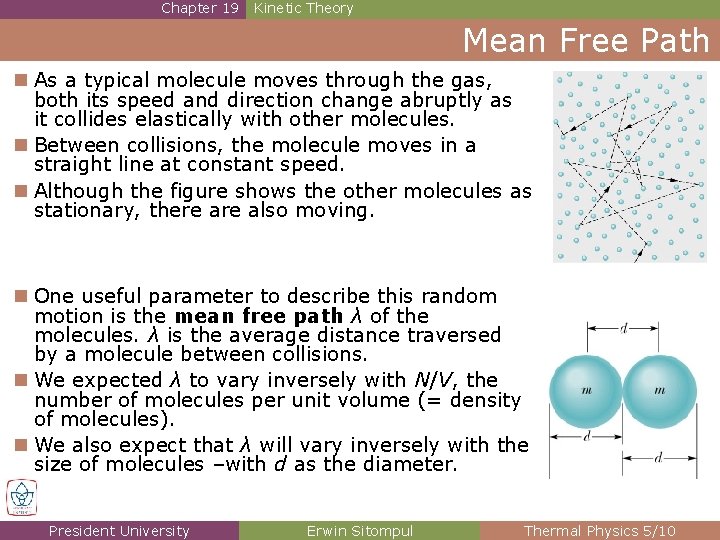
Chapter 19 Kinetic Theory Mean Free Path n As a typical molecule moves through the gas, both its speed and direction change abruptly as it collides elastically with other molecules. n Between collisions, the molecule moves in a straight line at constant speed. n Although the figure shows the other molecules as stationary, there also moving. n One useful parameter to describe this random motion is the mean free path λ of the molecules. λ is the average distance traversed by a molecule between collisions. n We expected λ to vary inversely with N/V, the number of molecules per unit volume (= density of molecules). n We also expect that λ will vary inversely with the size of molecules –with d as the diameter. President University Erwin Sitompul Thermal Physics 5/10

Chapter 19 Kinetic Theory Mean Free Path n The expression for the mean free path turns out to be: Mean Free Path n The mean free path of air molecules at sea level is about 0. 1 μm. n At an altitude of 100 km, with lower density of air, the mean free path rises to about 16 cm. n At 300 km, the mean free path is about 20 km. President University Erwin Sitompul Thermal Physics 5/11

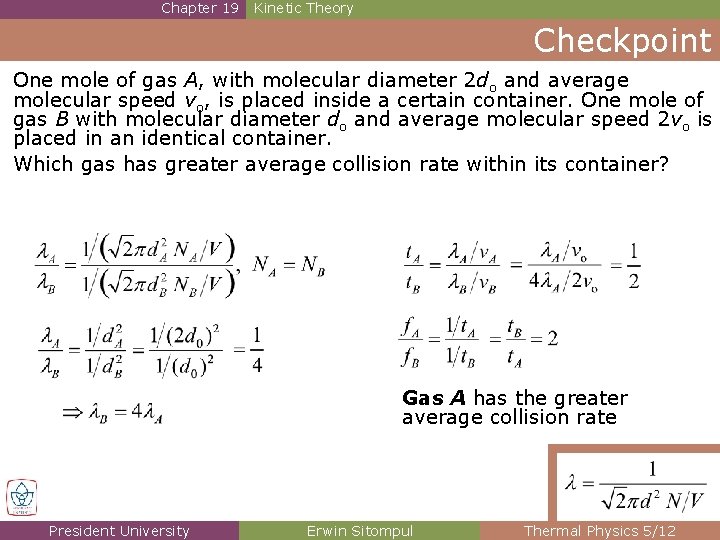
Chapter 19 Kinetic Theory Checkpoint One mole of gas A, with molecular diameter 2 do and average molecular speed vo, is placed inside a certain container. One mole of gas B with molecular diameter do and average molecular speed 2 vo is placed in an identical container. Which gas has greater average collision rate within its container? Gas A has the greater average collision rate President University Erwin Sitompul Thermal Physics 5/12

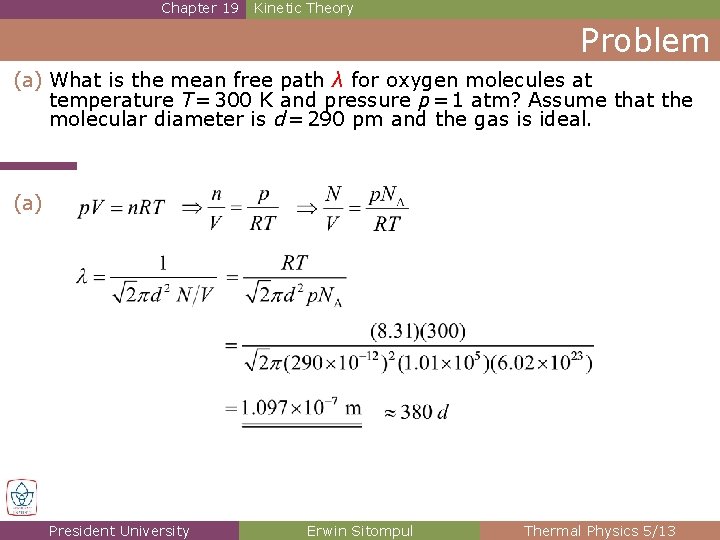
Chapter 19 Kinetic Theory Problem (a) What is the mean free path λ for oxygen molecules at temperature T = 300 K and pressure p = 1 atm? Assume that the molecular diameter is d = 290 pm and the gas is ideal. (a) President University Erwin Sitompul Thermal Physics 5/13

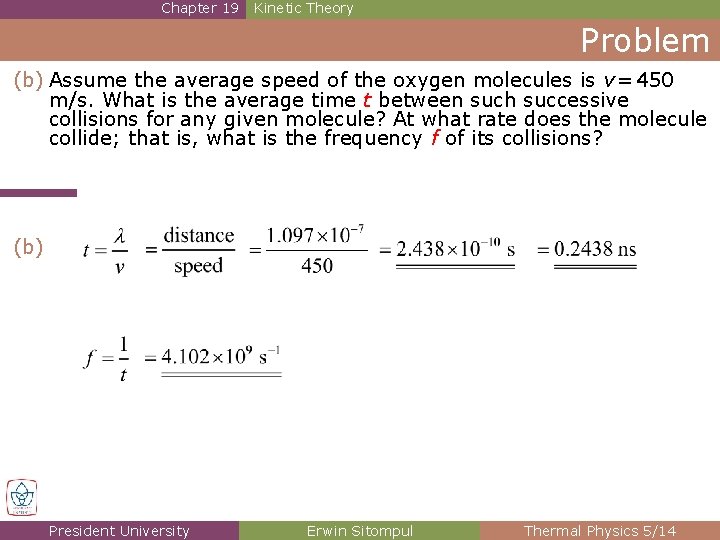
Chapter 19 Kinetic Theory Problem (b) Assume the average speed of the oxygen molecules is v = 450 m/s. What is the average time t between such successive collisions for any given molecule? At what rate does the molecule collide; that is, what is the frequency f of its collisions? (b) President University Erwin Sitompul Thermal Physics 5/14

Chapter 19 Kinetic Theory Class Group Assignments 1. How many moles of air must escape from a 10 m × 8 m × 5 m room when the temperature is raised from 0°C to 20°C? Assume the pressure remains unchanged at one atmosphere while the room is heated. (a) 1. 3 × 103 moles (d) 1. 2 × 103 moles (b) 7. 5 × 102 moles (e) 3. 7 × 102 moles (c) 1. 4 × 102 moles 2. Two ideal gases, X and Y, are thoroughly mixed and at thermal equilibrium in a single container. The molecular mass of X is 9 times that of Y. What is the ratio of root-mean-square velocities of the two gases, vrms, X/vrms, Y? (a) 9/1 (b) 3/1 (c) 1/3 (d) 1/9 (e) 1/1 3. The rms speed of an oxygen molecule at 0°C is 460 m/s. If the molar mass of oxygen is 32 g and that of helium is 4 g, then the rms speed of a helium molecule at 0°C is: (a) 230 m/s (b) 326 m/s (c) 650 m/s (d) 920 m/s (e) 1300 m/s 4. In a certain gas the molecules are 5. 0× 10– 9 m apart on average, have a mean free path of 5. 0× 10– 6 m, and have an average speed of 500 m/s. The rate at which a molecule has collisions with other molecules is about: (a) 10– 11 s– 1 (b) 10– 8 s– 1 (c) 1 s– 1 (d) 108 s– 1 (e) 1011 s– 1 President University Erwin Sitompul Thermal Physics 5/15

Chapter 19 Kinetic Theory Homework 5 1. (19 -22) The temperature and pressure in the Sun’s atmosphere are 2. 00× 10 6 K and 0. 0300 Pa. Calculate the rms speed of free electrons (mass 9. 11× 10 – 31 kg) there, assuming they are an ideal gas. 2. (19 -32) At 20°C and 750 torr pressure, the mean free paths for argon gas (Ar) and nitrogen gas (N 2) are λAr = 9. 9× 10– 6 cm and λN 2 = 27. 5× 10– 6 cm. (a) Find the ratio of the diameter of an Ar atom to that of an N 2 molecule. (b) What is the mean free path of argon at 20°C and 150 torr? (c) What is the mean free path of argon at – 40°C and 750 torr? n Deadline: Wednesday, 14 May 2014. n At the beginning of the make-up class due to Waisak Holiday. President University Erwin Sitompul Thermal Physics 5/16