The Sun crossed the celestial equator heading south








- Slides: 42

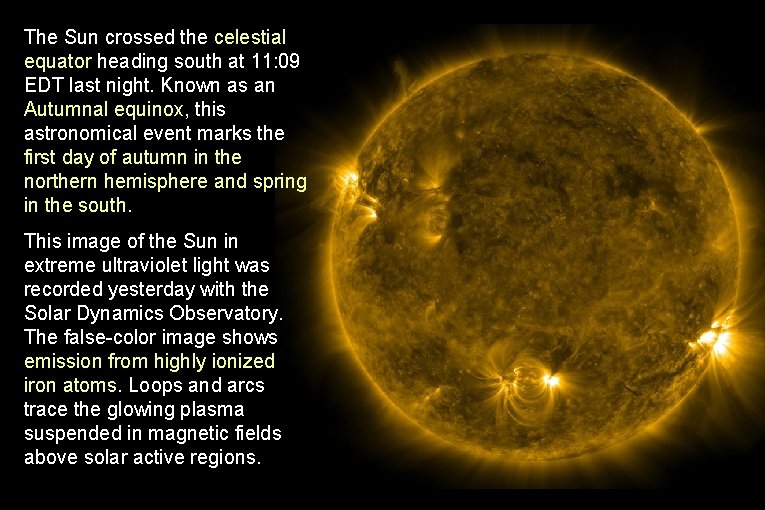
The Sun crossed the celestial equator heading south at 11: 09 EDT last night. Known as an Autumnal equinox, this astronomical event marks the first day of autumn in the northern hemisphere and spring in the south. This image of the Sun in extreme ultraviolet light was recorded yesterday with the Solar Dynamics Observatory. The false-color image shows emission from highly ionized iron atoms. Loops and arcs trace the glowing plasma suspended in magnetic fields above solar active regions.

Homework #3 is due Tuesday, Sept. 28, 5: 00 pm – extra credit question due at class time that day Exam #1, Thursday, Sept. 30 Review session: Tuesday (9/28), 7: 00 pm.

Light as a Particle v Light can also be treated as photons – packets of energy. v The energy carried by each photon depends on its frequency (color) v Energy: E = hf = hc/ [“h” is called Planck’s Constant] Shorter wavelength light carries more energy per photon.

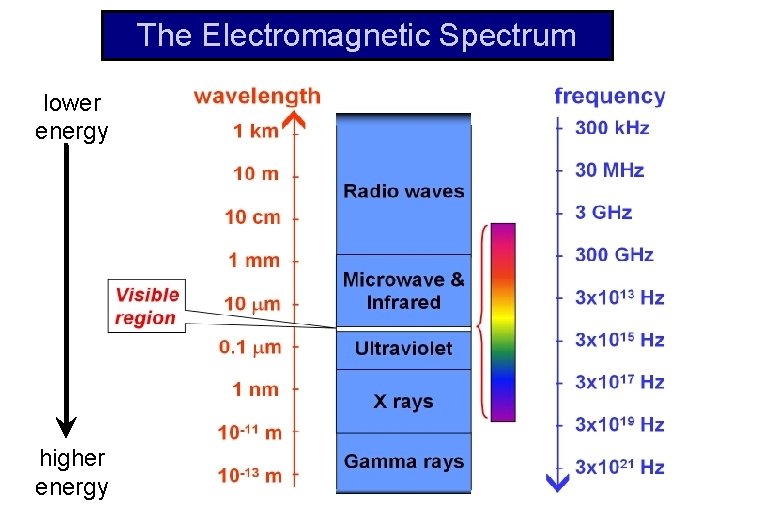
The Electromagnetic Spectrum lower energy higher energy

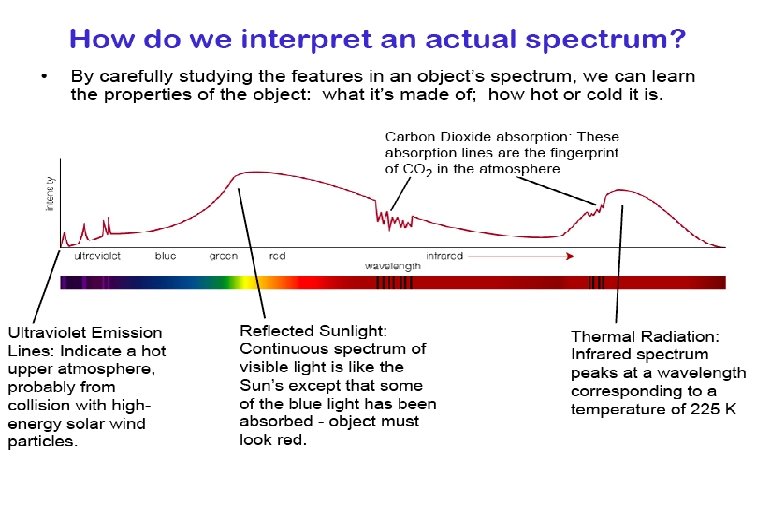
Light as Information Bearer Spectrum: light separated into its different wavelengths. Spectroscopy: The quantitative analysis of spectra The spectrum of an object can reveal the object’s: Composition Temperature Velocity

Four Ways in Which Light can Interact with Matter 1. emission – matter releases energy as light 2. absorption – matter takes energy from light 3. transmission – matter allows light to pass through it 4. reflection – matter reflects light The type of interaction is determined by characteristics of the “matter” and the wavelength of light.

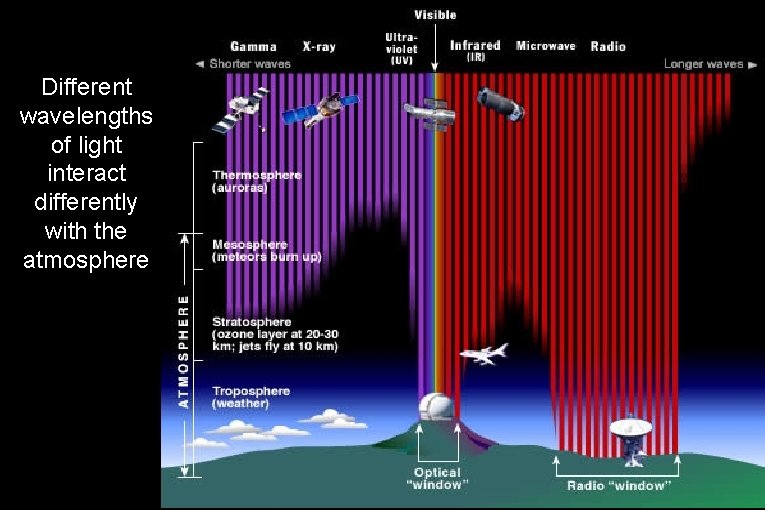
Different wavelengths of light interact differently with the atmosphere

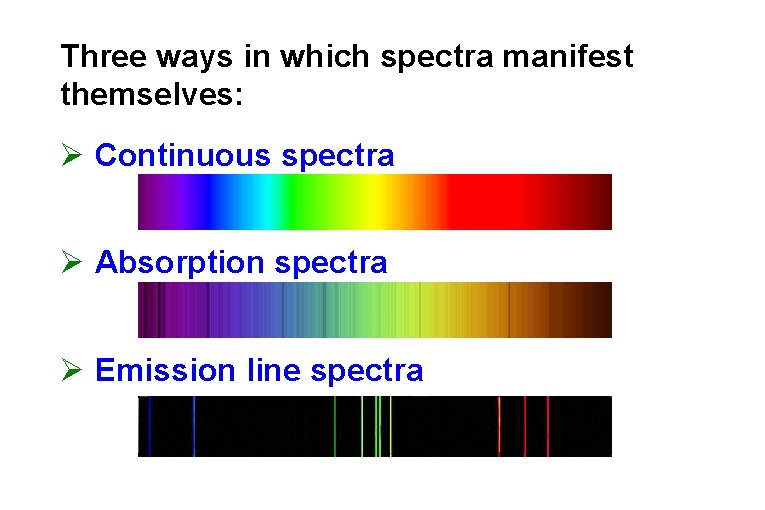
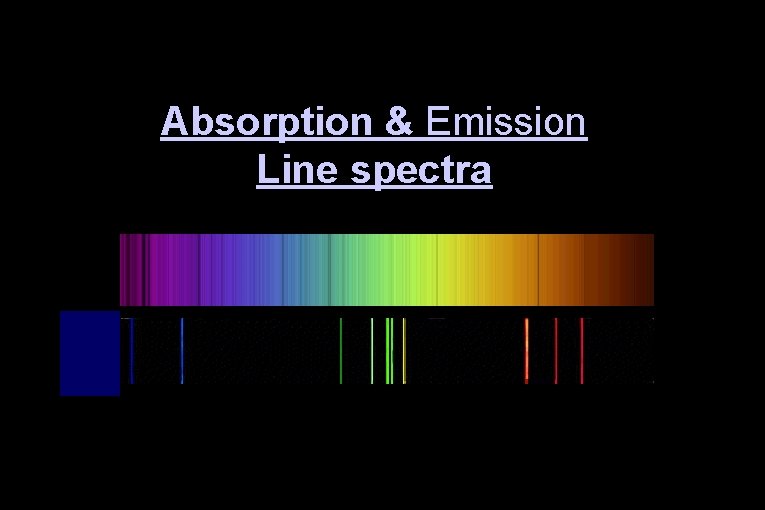
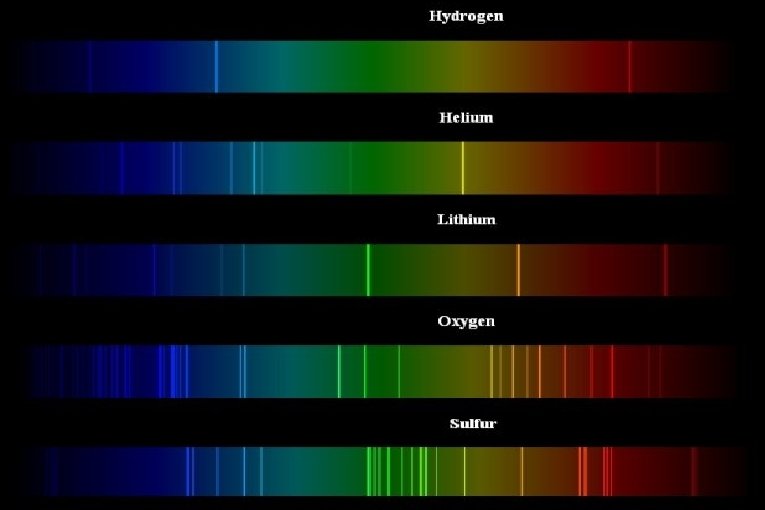
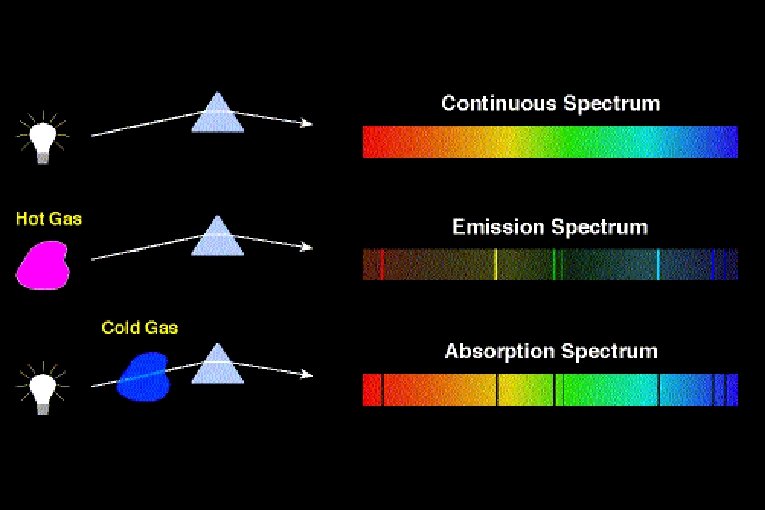
Three ways in which spectra manifest themselves: Ø Continuous spectra Ø Absorption spectra Ø Emission line spectra

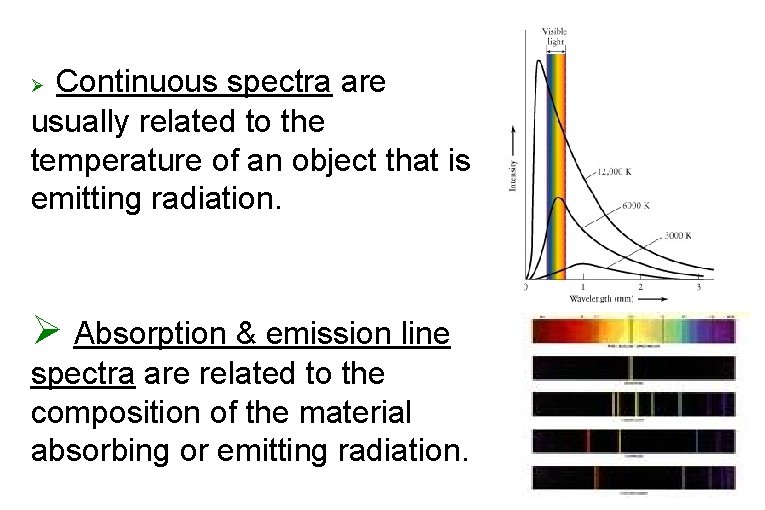
Continuous spectra are usually related to the temperature of an object that is emitting radiation. Ø Ø Absorption & emission line spectra are related to the composition of the material absorbing or emitting radiation.

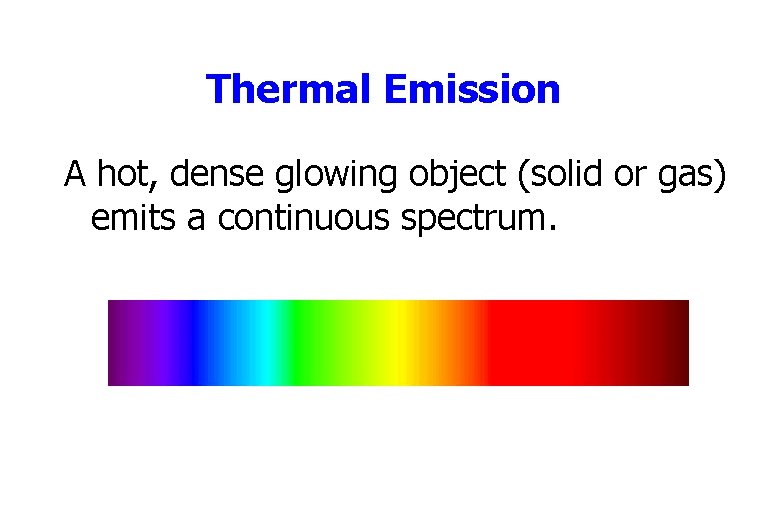
Thermal Emission A hot, dense glowing object (solid or gas) emits a continuous spectrum.

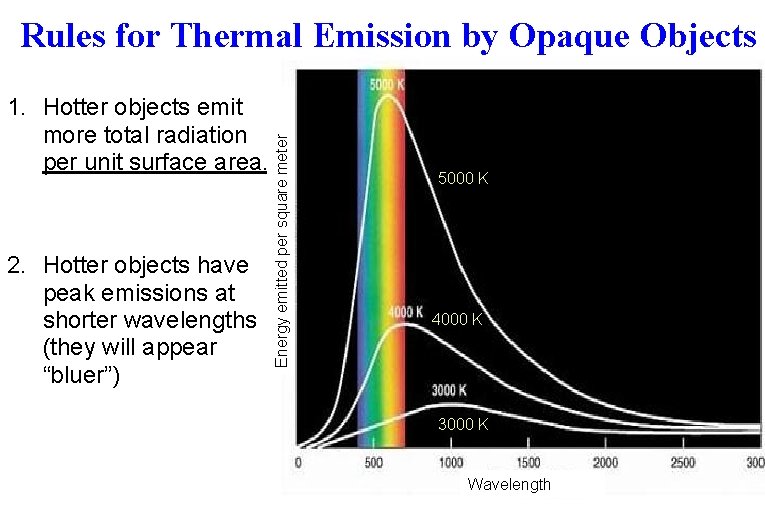
1. Hotter objects emit more total radiation per unit surface area. 2. Hotter objects have peak emissions at shorter wavelengths (they will appear “bluer”) Energy emitted per square meter Rules for Thermal Emission by Opaque Objects 5000 K 4000 K 3000 K Wavelength

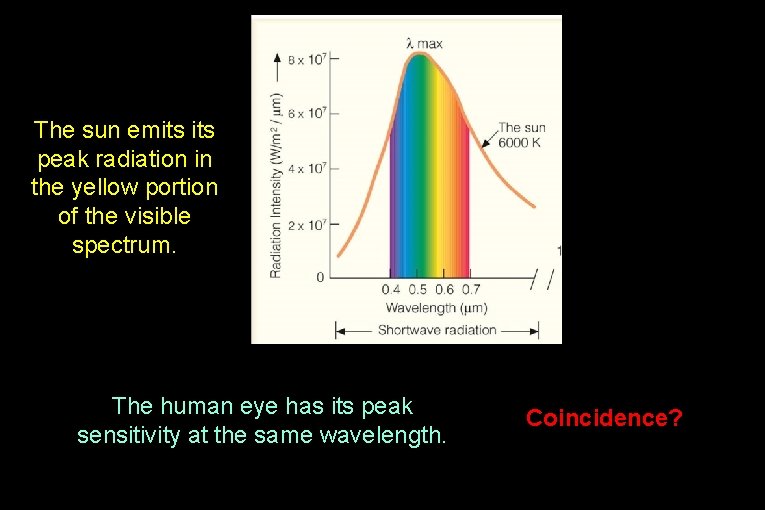
The sun emits peak radiation in the yellow portion of the visible spectrum. The human eye has its peak sensitivity at the same wavelength. Coincidence?

infrared At “room temperature”, or “body-temperature”, objects emit their peak radiation in the infrared. The surface of the Earth emits radiation in the infrared. visible Extremely hot objects will emit most of their radiation in the ultraviolet, x-ray or even the gamma ray portion of the spectrum

“Matter” and Light

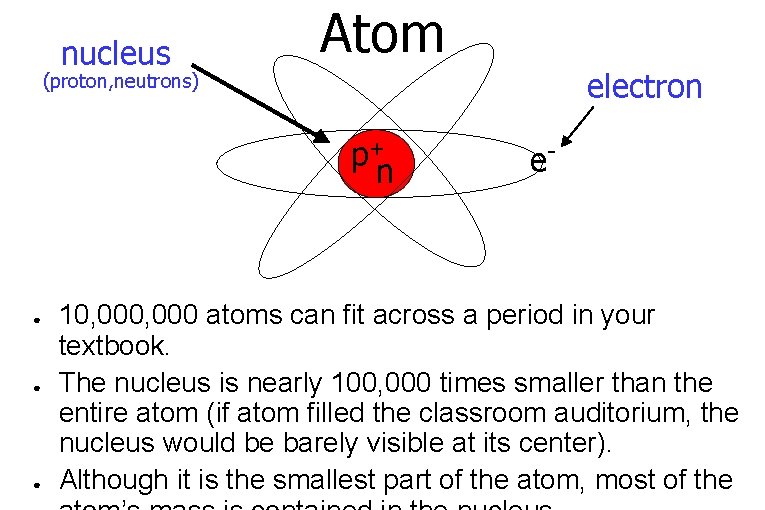
nucleus Atom electron (proton, neutrons) p+ n ● ● ● e- 10, 000 atoms can fit across a period in your textbook. The nucleus is nearly 100, 000 times smaller than the entire atom (if atom filled the classroom auditorium, the nucleus would be barely visible at its center). Although it is the smallest part of the atom, most of the

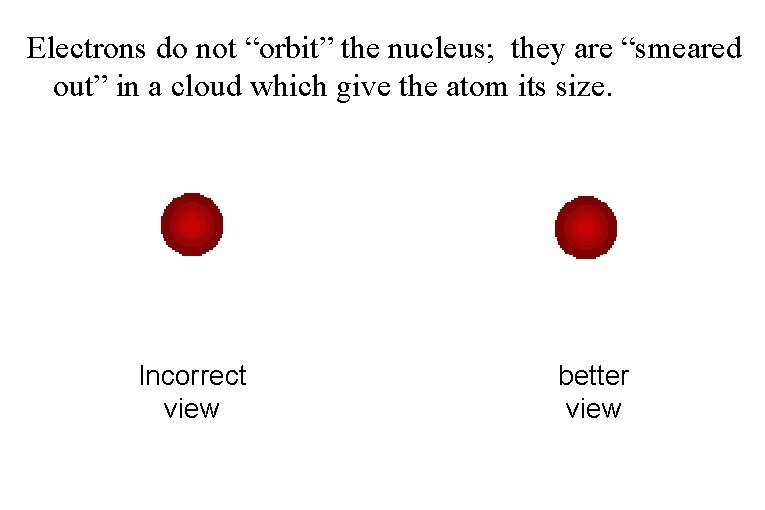
Electrons do not “orbit” the nucleus; they are “smeared out” in a cloud which give the atom its size. Incorrect view better view

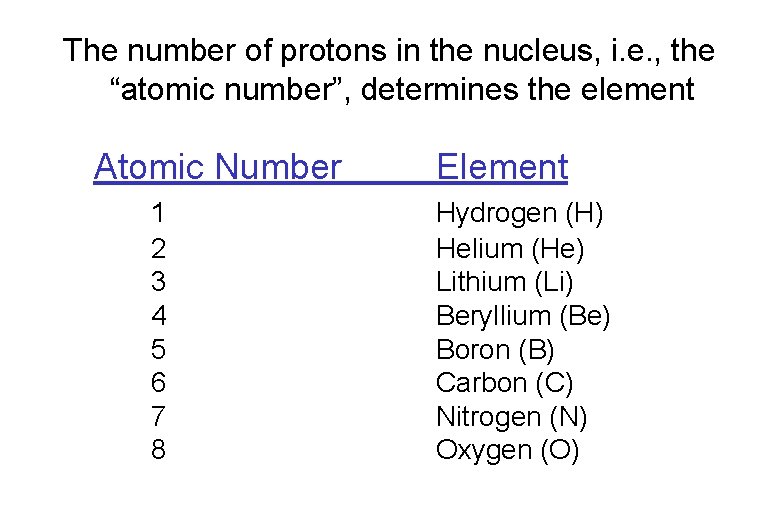
The number of protons in the nucleus, i. e. , the “atomic number”, determines the element Atomic Number 1 2 3 4 5 6 7 8 Element Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron (B) Carbon (C) Nitrogen (N) Oxygen (O)

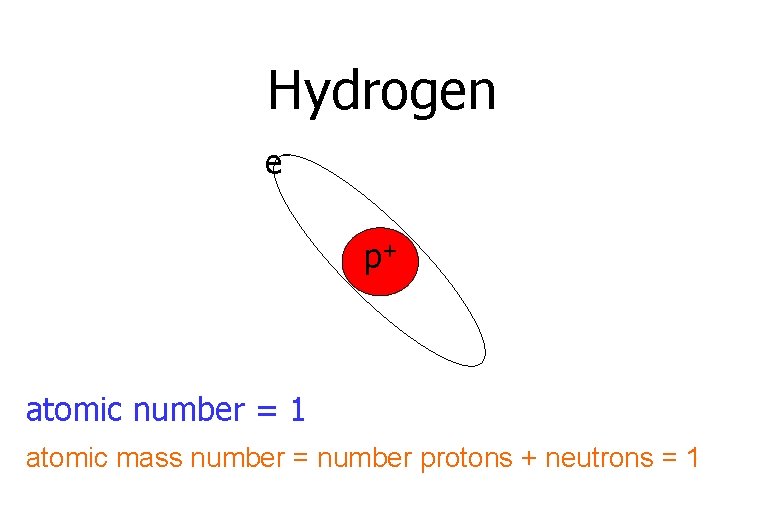
Hydrogen ep+ atomic number = 1 atomic mass number = number protons + neutrons = 1

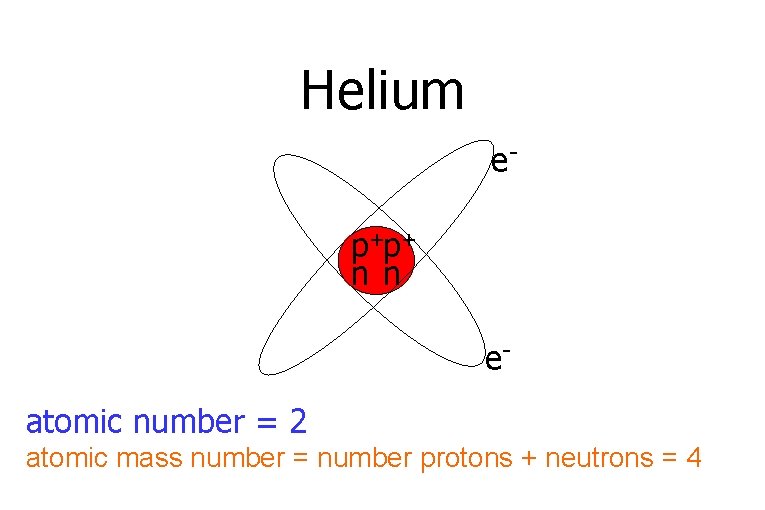
Helium ep+p+ n n eatomic number = 2 atomic mass number = number protons + neutrons = 4

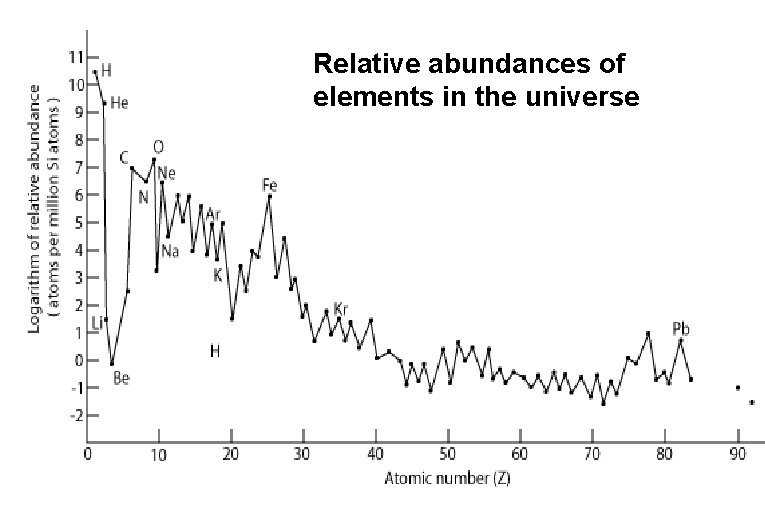
Relative abundances of elements in the universe

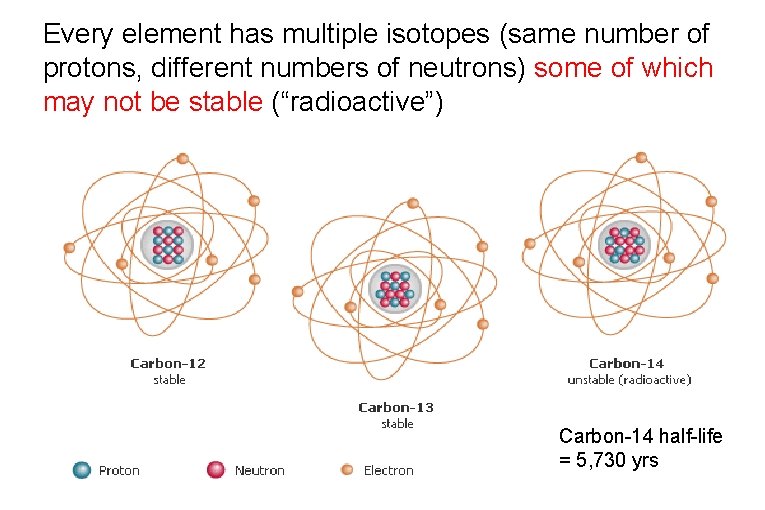
Every element has multiple isotopes (same number of protons, different numbers of neutrons) some of which may not be stable (“radioactive”) Carbon-14 half-life = 5, 730 yrs

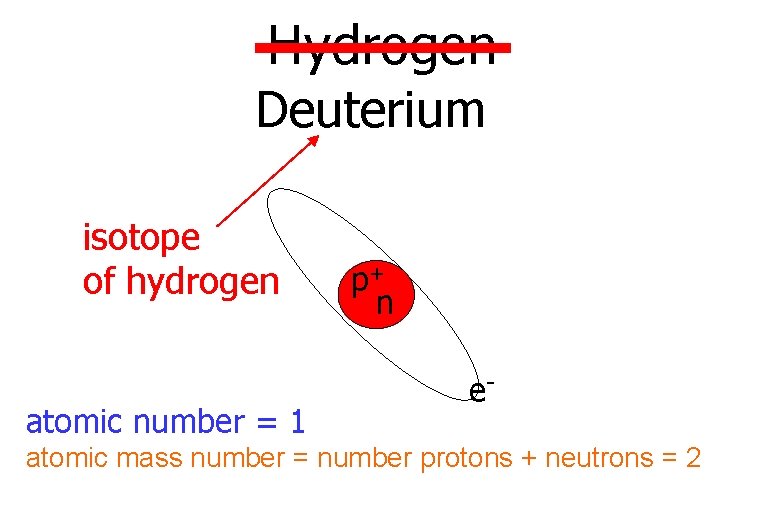
Hydrogen Deuterium isotope of hydrogen atomic number = 1 p+ n e- atomic mass number = number protons + neutrons = 2

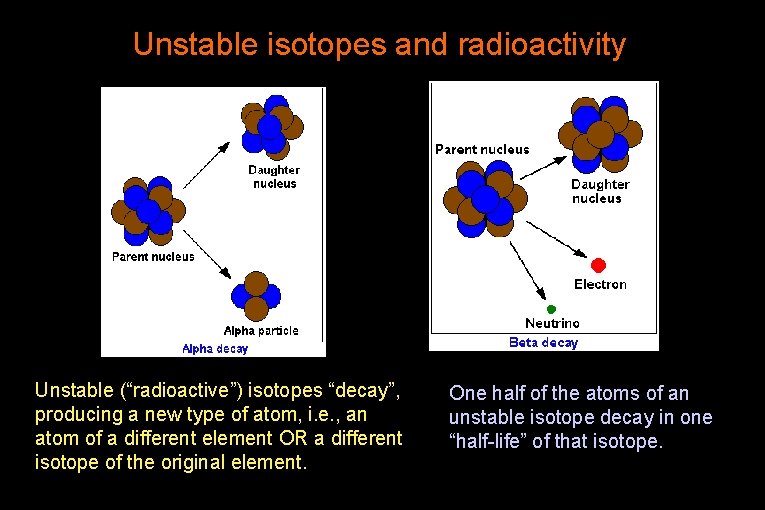
Unstable isotopes and radioactivity Unstable (“radioactive”) isotopes “decay”, producing a new type of atom, i. e. , an atom of a different element OR a different isotope of the original element. One half of the atoms of an unstable isotope decay in one “half-life” of that isotope.

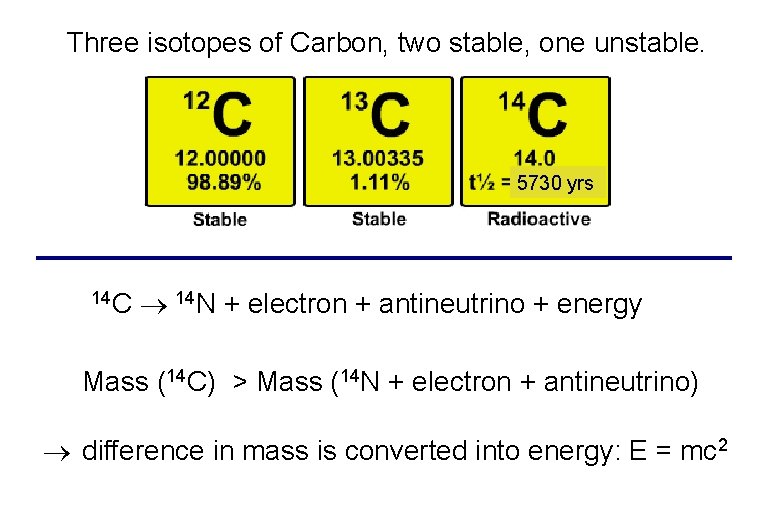
Three isotopes of Carbon, two stable, one unstable. 5730 yrs 14 C 14 N + electron + antineutrino + energy Mass (14 C) > Mass (14 N + electron + antineutrino) difference in mass is converted into energy: E = mc 2

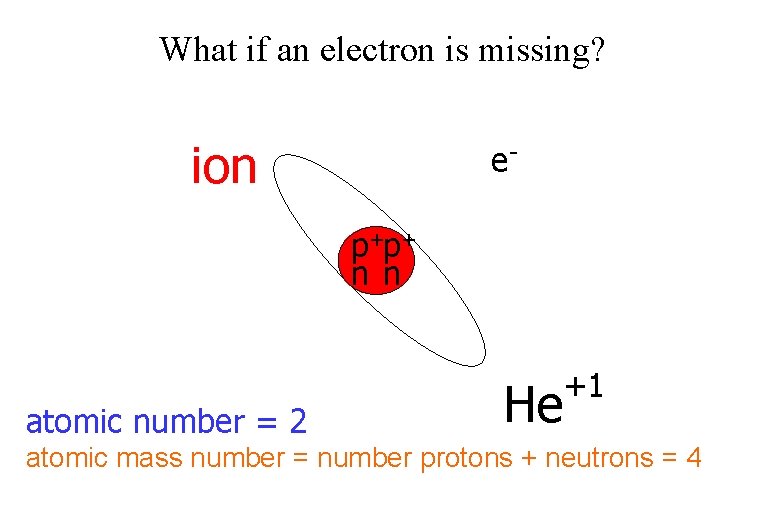
What if an electron is missing? ion ep+p+ n n atomic number = 2 He +1 atomic mass number = number protons + neutrons = 4

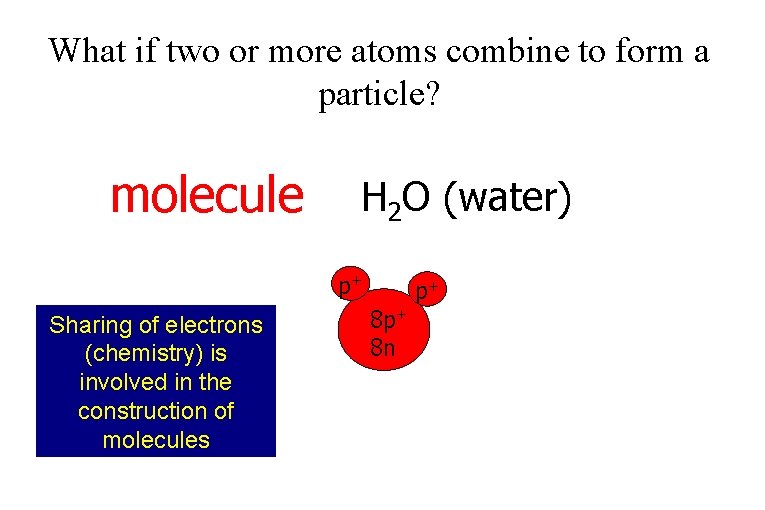
What if two or more atoms combine to form a particle? molecule H 2 O (water) p+ Sharing of electrons (chemistry) is involved in the construction of molecules 8 p+ 8 n p+

Absorption & Emission Line spectra

Electron Energy Levels ● ● ● Electrons cannot have just any energy while orbiting the nucleus. Only certain energy values are allowed (like the floors of an aprtment building). Electrons may only gain or lose certain specific amounts of energy (equal to differences in energy levels).

Electron Orbits / Absorption & Emission ● Electrons can gain or lose energy while they orbit the nucleus. ● When electrons have the lowest energy possible, we say the atom is in the ground state. ● When electrons have more energy than this, we say the atom is in an excited state. ● When electrons gain enough energy to escape the nucleus, we say the atom is ionized.

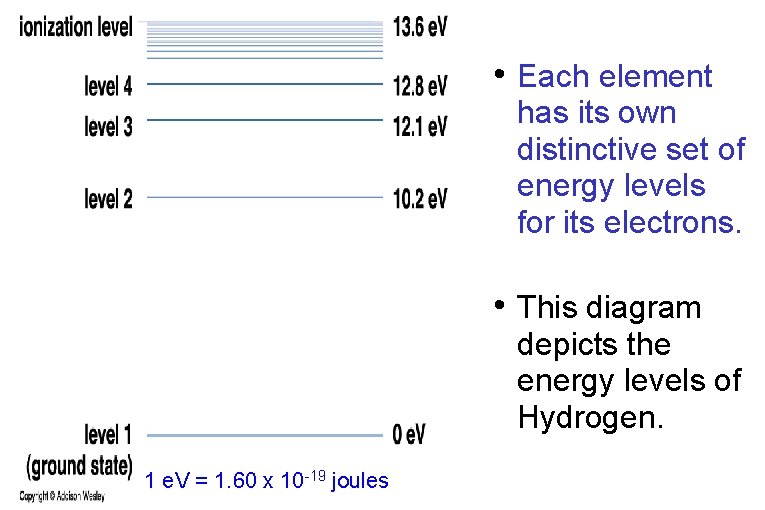
• Each element has its own distinctive set of energy levels for its electrons. • This diagram depicts the energy levels of Hydrogen. 1 e. V = 1. 60 x 10 -19 joules

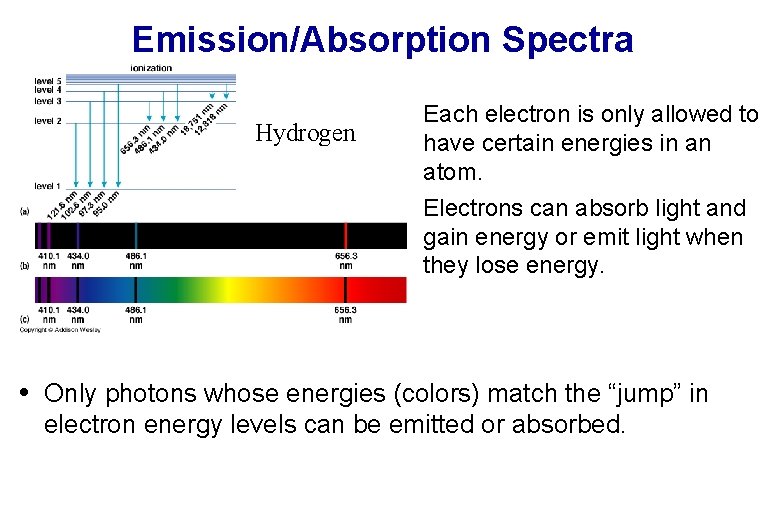
Emission/Absorption Spectra Hydrogen • Each electron is only allowed to have certain energies in an atom. • Electrons can absorb light and gain energy or emit light when they lose energy. • Only photons whose energies (colors) match the “jump” in electron energy levels can be emitted or absorbed.

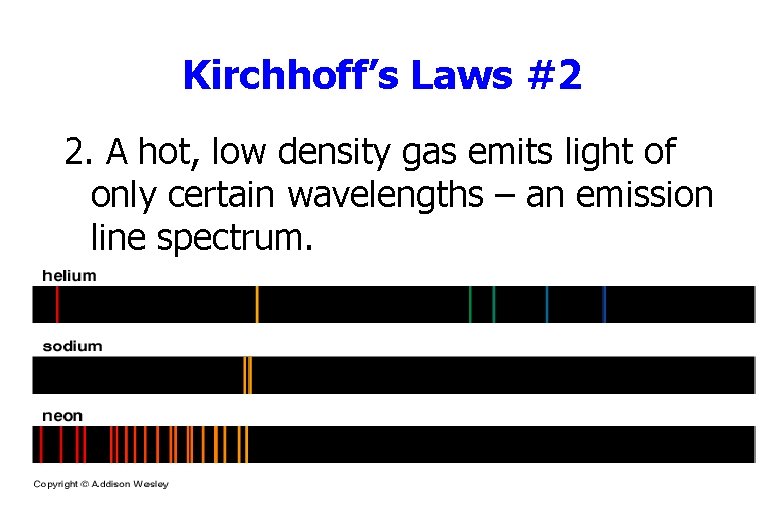
Kirchhoff’s Laws #2 2. A hot, low density gas emits light of only certain wavelengths – an emission line spectrum.


Kirchhoff’s Law #3 3. When light having a continuous spectrum passes through a cool gas, dark lines appear in the continuous spectrum – an absorption line spectrum.

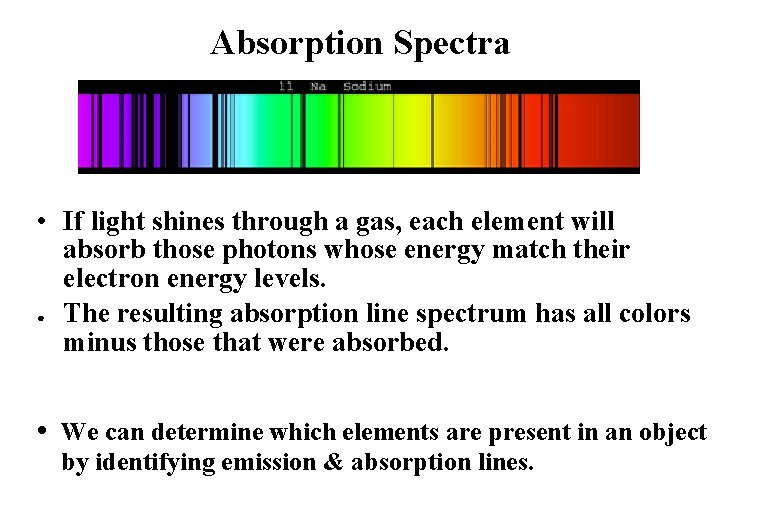
Absorption Spectra • If light shines through a gas, each element will absorb those photons whose energy match their electron energy levels. ● The resulting absorption line spectrum has all colors minus those that were absorbed. • We can determine which elements are present in an object by identifying emission & absorption lines.

Molecules have rotational & vibrational energy levels (less energetic than electron energy levels, energies correspond with infrared, microwave, and radio radiations)



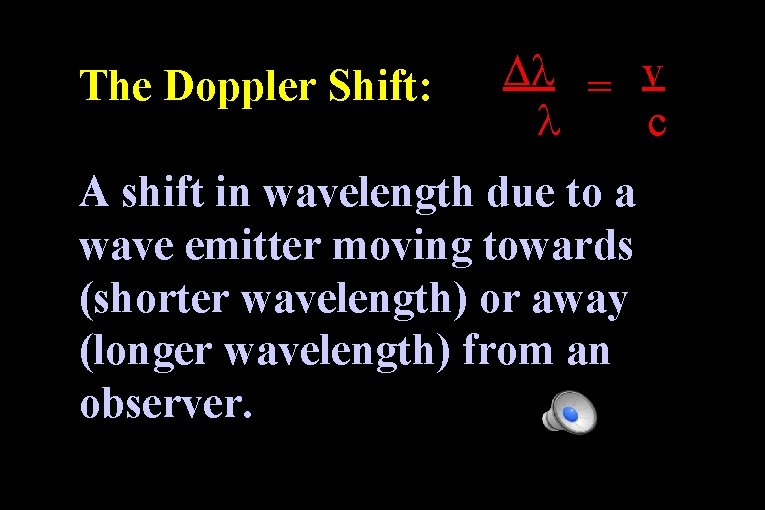
The Doppler Shift: = v c A shift in wavelength due to a wave emitter moving towards (shorter wavelength) or away (longer wavelength) from an observer.

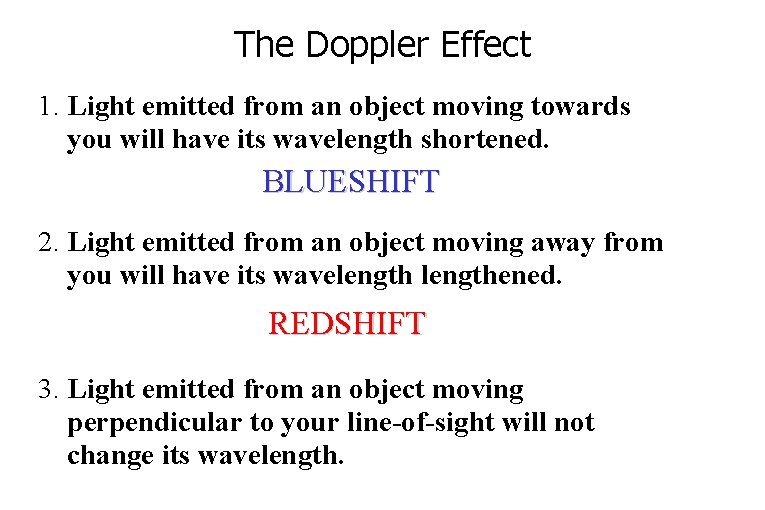
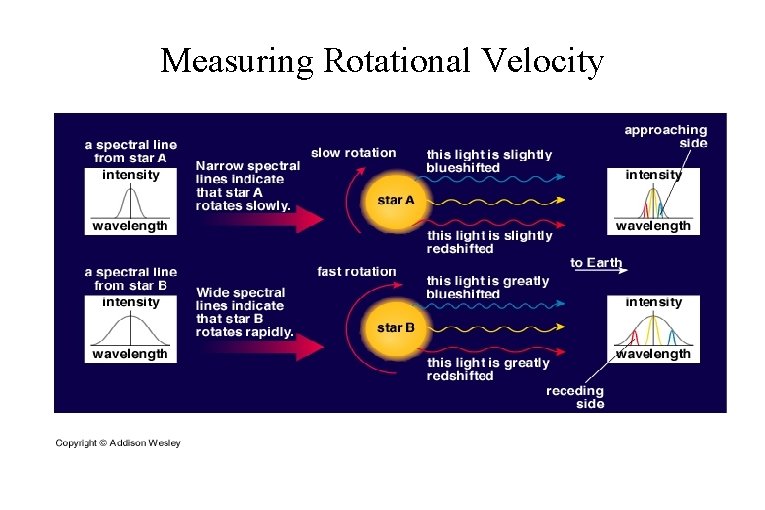
The Doppler Effect 1. Light emitted from an object moving towards you will have its wavelength shortened. BLUESHIFT 2. Light emitted from an object moving away from you will have its wavelengthened. REDSHIFT 3. Light emitted from an object moving perpendicular to your line-of-sight will not change its wavelength.

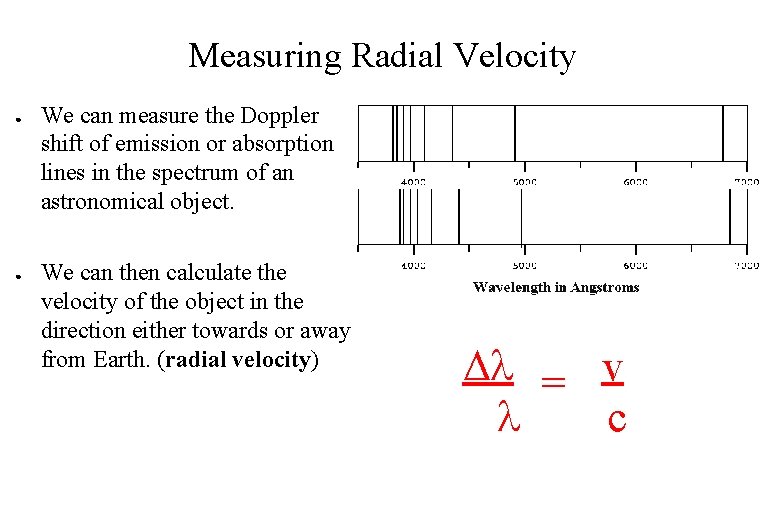
Measuring Radial Velocity ● ● We can measure the Doppler shift of emission or absorption lines in the spectrum of an astronomical object. We can then calculate the velocity of the object in the direction either towards or away from Earth. (radial velocity) = v c

Measuring Rotational Velocity