The Aerosol Microphysics Model M 7 IACETH ECHAMHAM






- Slides: 15

The Aerosol Microphysics Model M 7 IACETH ECHAM/HAM presentation series Nr. 2, 24. 02. 2010

M 7: background and short history Ø M 7 was conceived as a model capable of simulating the time evolution of a multi-component, size-resolved aerosol population Ø Originally 0 -D (a box model) Ø First developed at JRC, Ispra by J. Wilson and E. Vignati with contributions from J. Feichter (MPI-M, Hamburg) Ø Adapted to 3 dimensions and set into the ECHAM 5/HAM framework by P. Stier as part of his Ph. D work Ø Further developments: new nucleation schemes (J. Kazil, 2007 -2008), new SO 4 condensation scheme (J. Kazil, 2007), new water uptake scheme (D. O’Donnell, 2008), greater flexibility with respect to included species (D. O’Donnell 2009), some optimisation for scalar machines (D. O’Donnell 2009). Ø Basic aerosol dynamics scheme unchanged sinception

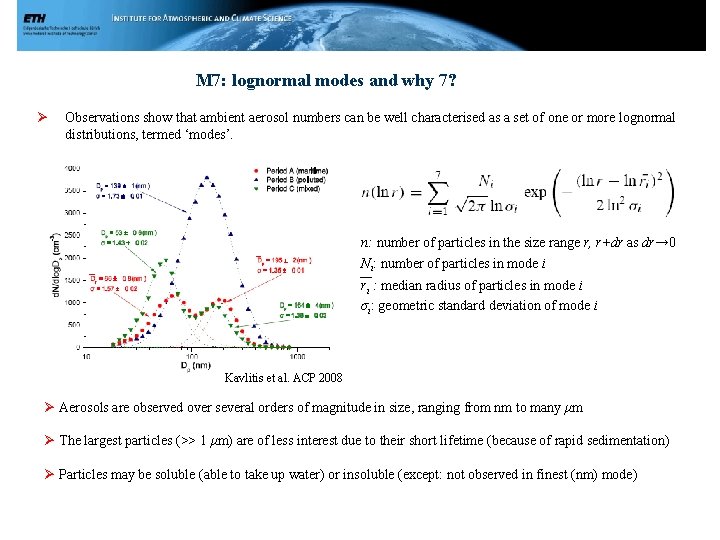
M 7: lognormal modes and why 7? Ø Observations show that ambient aerosol numbers can be well characterised as a set of one or more lognormal distributions, termed ‘modes’. n: number of particles in the size range r, r+dr as dr→ 0 Ni: number of particles in mode i ri : median radius of particles in mode i σi: geometric standard deviation of mode i Kavlitis et al. ACP 2008 Ø Aerosols are observed over several orders of magnitude in size, ranging from nm to many μm Ø The largest particles (>> 1 μm) are of less interest due to their short lifetime (because of rapid sedimentation) Ø Particles may be soluble (able to take up water) or insoluble (except: not observed in finest (nm) mode)

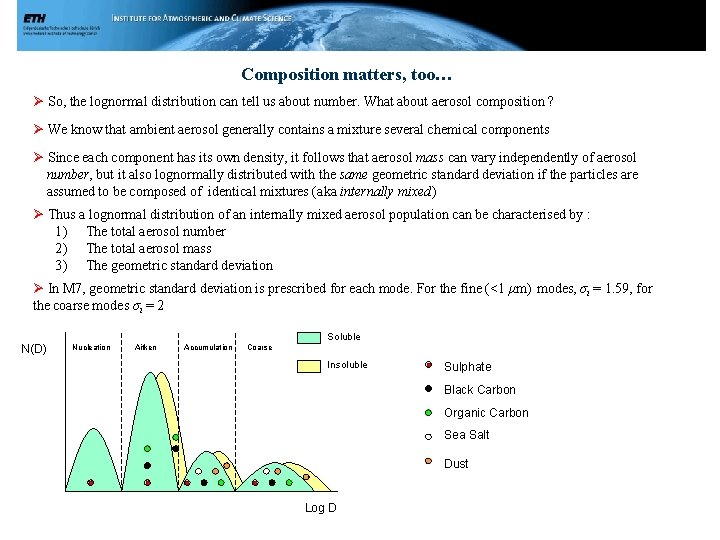
Composition matters, too… Ø So, the lognormal distribution can tell us about number. What about aerosol composition ? Ø We know that ambient aerosol generally contains a mixture several chemical components Ø Since each component has its own density, it follows that aerosol mass can vary independently of aerosol number, but it also lognormally distributed with the same geometric standard deviation if the particles are assumed to be composed of identical mixtures (aka internally mixed) Ø Thus a lognormal distribution of an internally mixed aerosol population can be characterised by : 1) The total aerosol number 2) The total aerosol mass 3) The geometric standard deviation Ø In M 7, geometric standard deviation is prescribed for each mode. For the fine (<1 μm) modes, σi = 1. 59, for the coarse modes σi = 2 Soluble N(D) Nucleation Aitken Accumulation Coarse Insoluble Sulphate Black Carbon Organic Carbon Sea Salt Dust Log D

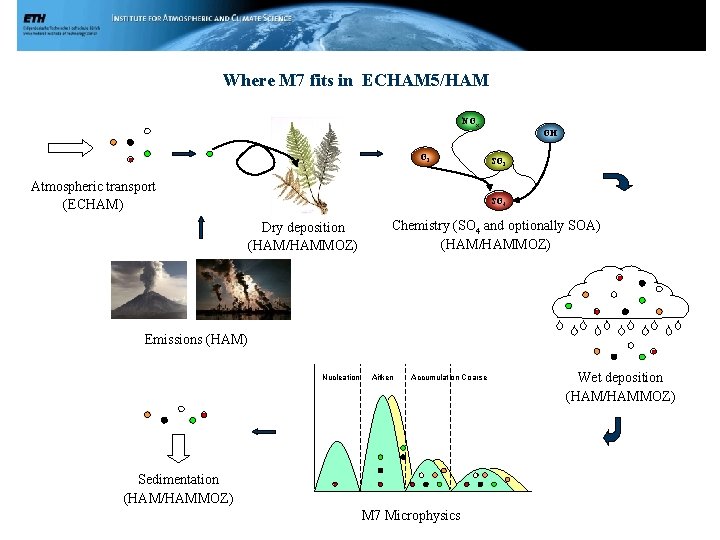
Where M 7 fits in ECHAM 5/HAM NOx OH O 3 Atmospheric transport (ECHAM) SO 2 SO 4 Dry deposition (HAM/HAMMOZ) Chemistry (SO 4 and optionally SOA) (HAM/HAMMOZ) Emissions (HAM) Nucleation Aitken Accumulation Coarse Sedimentation (HAM/HAMMOZ) M 7 Microphysics Wet deposition (HAM/HAMMOZ)

Processes modelled by M 7 Ø Calculation of the median dry particle radius per mode Ø Water uptake on soluble modes and hence the median wet particle radius per soluble mode Ø Condensation of gas phase sulphate Ø Nucleation of new particles Ø Ageing of insoluble particles (coating by sulphate and transfer to soluble modes) Ø Coagulation of particles Ø Particle growth (transfer of mass and number to larger modes)

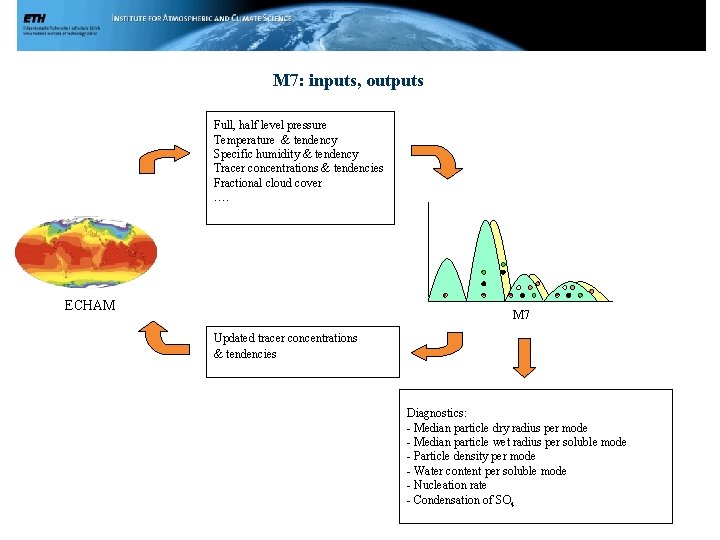
M 7: inputs, outputs Full, half level pressure Temperature & tendency Specific humidity & tendency Tracer concentrations & tendencies Fractional cloud cover …. ECHAM M 7 Updated tracer concentrations & tendencies Diagnostics: - Median particle dry radius per mode - Median particle wet radius per soluble mode - Particle density per mode - Water content per soluble mode - Nucleation rate - Condensation of SO 4

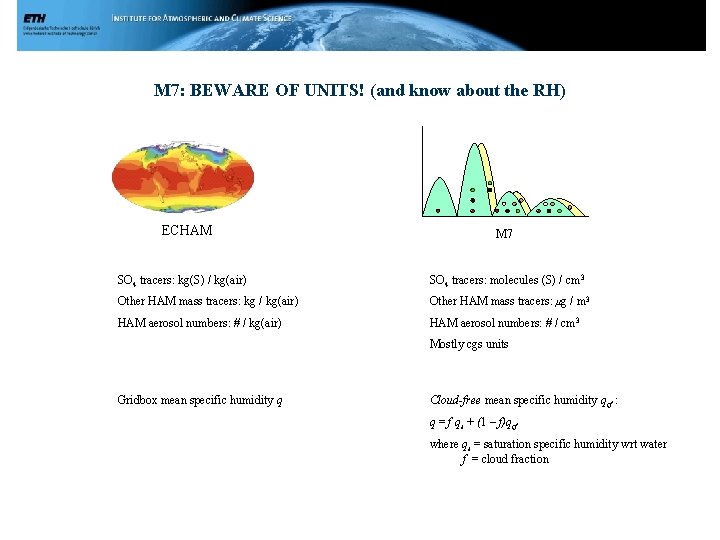
M 7: BEWARE OF UNITS! (and know about the RH) ECHAM M 7 SO 4 tracers: kg(S) / kg(air) SO 4 tracers: molecules (S) / cm 3 Other HAM mass tracers: kg / kg(air) Other HAM mass tracers: μg / m 3 HAM aerosol numbers: # / kg(air) HAM aerosol numbers: # / cm 3 Mostly cgs units Gridbox mean specific humidity q Cloud-free mean specific humidity qcf : q = f qs + (1 – f)qcf where qs = saturation specific humidity wrt water f = cloud fraction

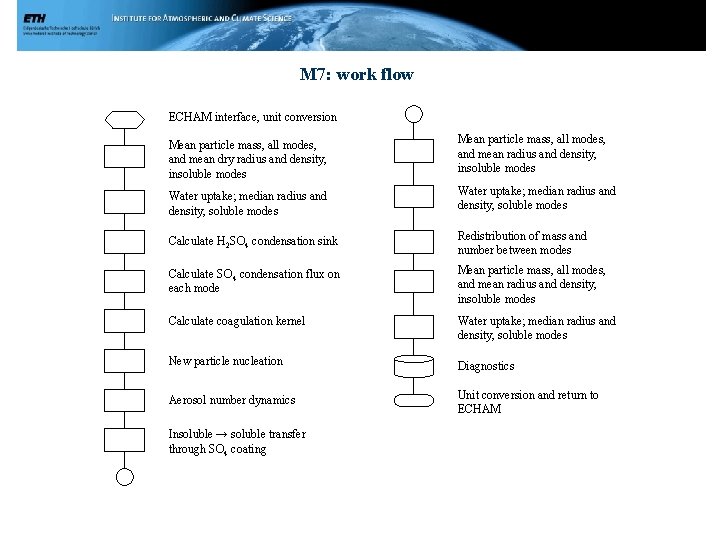
M 7: work flow ECHAM interface, unit conversion Mean particle mass, all modes, and mean dry radius and density, insoluble modes Mean particle mass, all modes, and mean radius and density, insoluble modes Water uptake; median radius and density, soluble modes Calculate H 2 SO 4 condensation sink Redistribution of mass and number between modes Calculate SO 4 condensation flux on each mode Mean particle mass, all modes, and mean radius and density, insoluble modes Calculate coagulation kernel Water uptake; median radius and density, soluble modes New particle nucleation Diagnostics Aerosol number dynamics Unit conversion and return to ECHAM Insoluble → soluble transfer through SO 4 coating

M 7 : water uptake Ø Two alternatives for water uptake on aerosols: 1) Petters and Kreidenweis, ACP 2007 (SO 4, SS, organics), aka Kappa-Köhler theory approach (default) 2) Vignati, Wilson and Stier (JGR 2004), sulphate, and Jacobson et al. (1996) for particles containing sea salt Alternative 1: Ø Petters and Kreidenweis showed that the hygroscopic growth factor (gf) of a multicomponent aerosol can be expressed as a function of temperature (T), relative humiidty (RH), dry diameter (Dd) and κ : Ø This is solved offline for gf using T, RH, κ and Dd of atmospheric relevance and the results stored in a lookup table (lut_kappa. nc). Ø In runtime, we first calculate the dry diameter Dd and the κ value for each soluble mode, then simply look up the growth factor to get the wet diameter (see: m 7_kappa, mo_aero_kappa) Alternative 2: Ø For particles with negliglible sea salt, a parameterisation of water uptake by sulphuric acid derived by J. Wilson is used (polynomial in log [SO 4], T, RH) (See: m 7_equiz, m 7_equimix) Ø For particles containing sea salt, the ZSR approach is applied: for calculation of binary molalities an empirical approach is also used (See: m 7_equil)

M 7: nucleation Ø Alternatives: i. nucleation of new particles from sulphuric acid and water (Vehkamaeki et al. , 2002) ii. neutral and charged nucleation of H 2 SO 4 and water (Lovejoy et al, 2004, Hanson and Lovejoy, 2006) iii. nucleation in the presence of organics (Kulmala et al. , 2006, Riipenen et al. 2007) Ø Vehkamaeki et al is based on classical nucleation theory, accounting for hydration. Known to give too low nucleation rates in the lower troposphere. Ø Neutral and charged nucleation scheme uses a lookup table with inputs T, RH, [H 2 SO 4], condensation sink strength of H 2 SO 4, and ionisation rate and output formation rate of clusters of ≥ 15 molecules. Now the default. Ø Ionisation rate is calculated as a function of galactic cosmic ray intensity, taking into account the solar cycle Ø Organic nucleation schemes actually depend on [H 2 SO 4] or [H 2 SO 4]2 rather than directly on the concentration of organics in the atmosphere in accordance with observations a Hyytiälä. The organics are supposed to be represented by a multiplicative constant. In M 7, this is applied only in the boundary layer in the forested fraction. Default = neither of the organic schemes

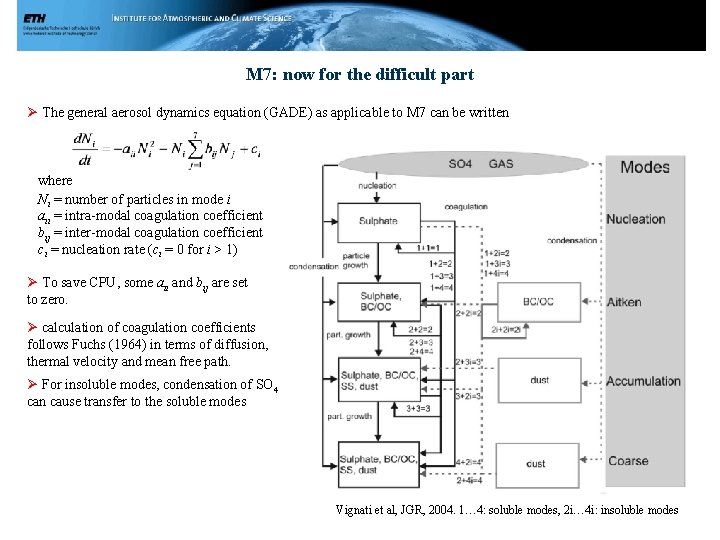
M 7: now for the difficult part Ø The general aerosol dynamics equation (GADE) as applicable to M 7 can be written where Ni = number of particles in mode i aii = intra-modal coagulation coefficient bij = inter-modal coagulation coefficient ci = nucleation rate (ci = 0 for i > 1) Ø To save CPU, some aii and bij are set to zero. Ø calculation of coagulation coefficients follows Fuchs (1964) in terms of diffusion, thermal velocity and mean free path. Ø For insoluble modes, condensation of SO 4 can cause transfer to the soluble modes Vignati et al, JGR, 2004. 1… 4: soluble modes, 2 i… 4 i: insoluble modes

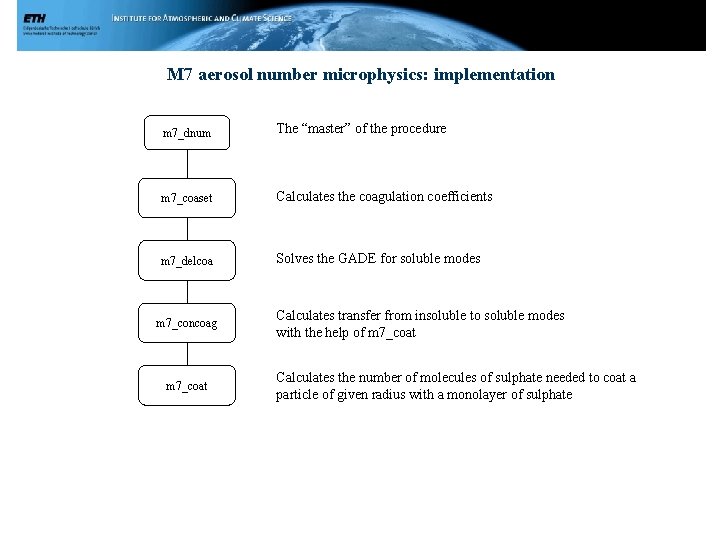
M 7 aerosol number microphysics: implementation m 7_dnum The “master” of the procedure m 7_coaset Calculates the coagulation coefficients m 7_delcoa Solves the GADE for soluble modes m 7_concoag m 7_coat Calculates transfer from insoluble to soluble modes with the help of m 7_coat Calculates the number of molecules of sulphate needed to coat a particle of given radius with a monolayer of sulphate

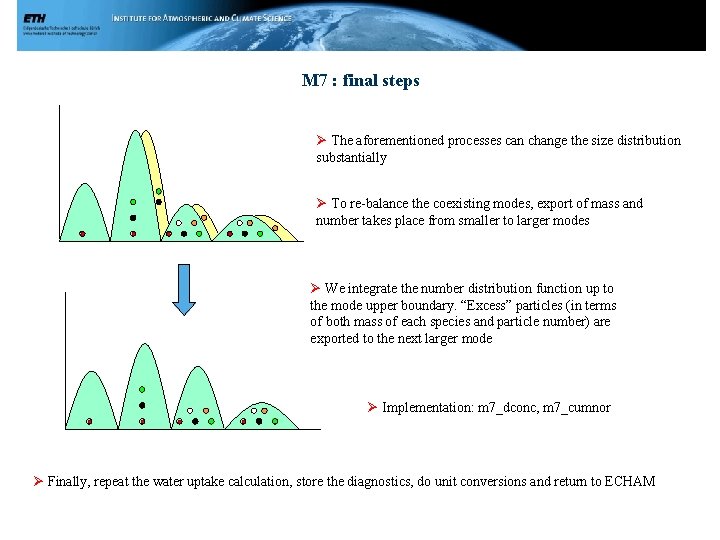
M 7 : final steps Ø The aforementioned processes can change the size distribution substantially Ø To re-balance the coexisting modes, export of mass and number takes place from smaller to larger modes Ø We integrate the number distribution function up to the mode upper boundary. “Excess” particles (in terms of both mass of each species and particle number) are exported to the next larger mode Ø Implementation: m 7_dconc, m 7_cumnor Ø Finally, repeat the water uptake calculation, store the diagnostics, do unit conversions and return to ECHAM

Thank you for your attention!