Study Design for the PARTNER 3 LowRisk Trial







- Slides: 13

Study Design for the PARTNER 3 Low-Risk Trial Martin B. Leon, MD 5 mins Columbia University Medical Center Cardiovascular Research Foundation New York City

Disclosure Statement of Financial Interest TVT 2016, Chicago, IL; June 15 -18, 2016 Martin B. Leon, MD Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation / Financial Relationship Company • Grant / Research Support / SAB • Abbott, Boston Scientific, Edwards Lifescience, Medtronic • Consulting Fees / Honoraria • None • Shareholder / Equity • Claret, GDS, Mitralign, Valve Medical

TAVR in Low-Risk Patients • TAVR is now the dominant therapy in inoperable and high-risk AS patients; recent data shows clear benefits in intermediate-risk patients as well! • As complications continue to decline and the procedure is further simplified, there are clear secondary benefits associated with TAVR – reduced ICU and hospital LOS, more rapid QOL recovery, lower frequency of AKI, bleeding, and post-operative AF, and improved valve hemodynamics. • TAVR should now be introduced to low-risk AS patients in thoughtful randomized clinical trials!

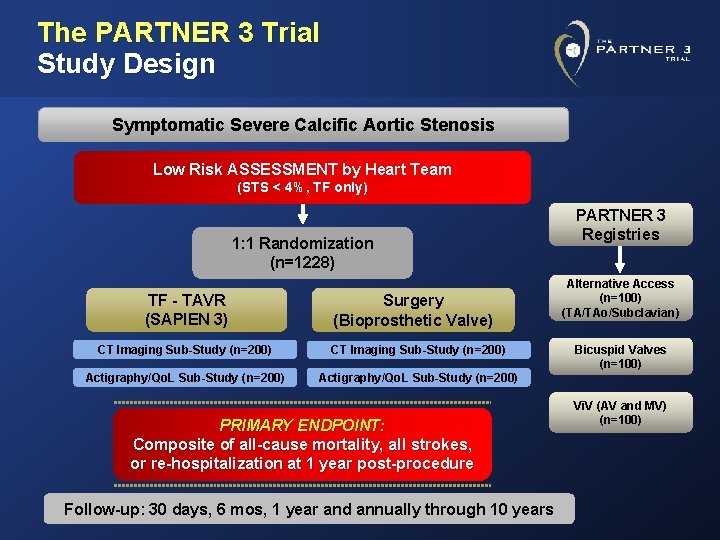
The PARTNER 3 Trial Study Design Symptomatic Severe Calcific Aortic Stenosis Low Risk ASSESSMENT by Heart Team (STS < 4%, TF only) 1: 1 Randomization (n=1228) TF - TAVR (SAPIEN 3) Surgery (Bioprosthetic Valve) CT Imaging Sub-Study (n=200) Actigraphy/Qo. L Sub-Study (n=200) PRIMARY ENDPOINT: Composite of all-cause mortality, all strokes, or re-hospitalization at 1 year post-procedure Follow-up: 30 days, 6 mos, 1 year and annually through 10 years PARTNER 3 Registries Alternative Access (n=100) (TA/TAo/Subclavian) Bicuspid Valves (n=100) Vi. V (AV and MV) (n=100)

The PARTNER 3 Trial Key Inclusion Criteria • Severe, calcific aortic stenosis Ø AVA ≤ 1. 0 cm 2 or AVA index ≤ 0. 6 cm 2/m 2 Ø Mean gradient ≥ 40 mm. Hg or jet velocity ≥ 4. 0 m/sec • Symptom status Ø NYHA Functional Class ≥ 2, or Ø Exercise test with reduced exercise capacity, abnormal BP response, or arrhythmias, or Ø Asymptomatic with LVEF < 50% • Heart Team agrees patient is low operative risk and STS < 4% • Informed consent in all patients

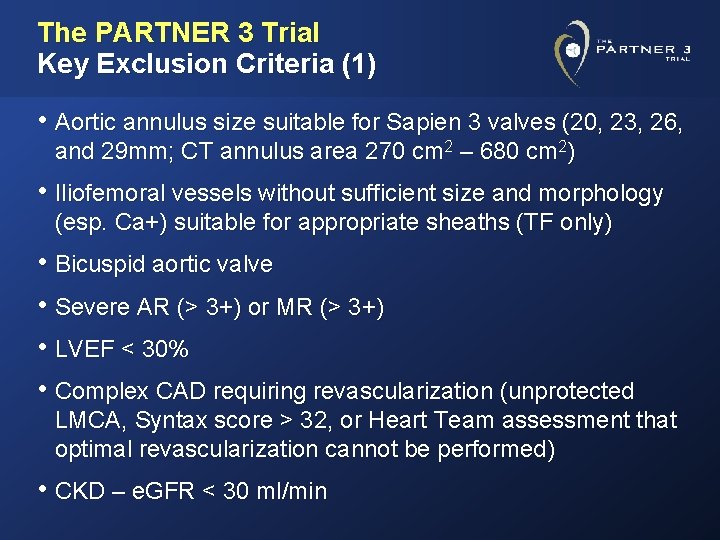
The PARTNER 3 Trial Key Exclusion Criteria (1) • Aortic annulus size suitable for Sapien 3 valves (20, 23, 26, and 29 mm; CT annulus area 270 cm 2 – 680 cm 2) • Iliofemoral vessels without sufficient size and morphology (esp. Ca+) suitable for appropriate sheaths (TF only) • Bicuspid aortic valve • Severe AR (> 3+) or MR (> 3+) • LVEF < 30% • Complex CAD requiring revascularization (unprotected LMCA, Syntax score > 32, or Heart Team assessment that optimal revascularization cannot be performed) • CKD – e. GFR < 30 ml/min

The PARTNER 3 Trial Key Exclusion Criteria (2) • MI within 30 days, stroke/TIA within 90 days • Severe lung disease (FEV 1 < 50% predicted) or home oxygen • Significant frailty as determined by the Heart Team (after objective assessments) • Hemodynamic or respiratory instability requiring inotropic support or mechanical ventilation within 30 days

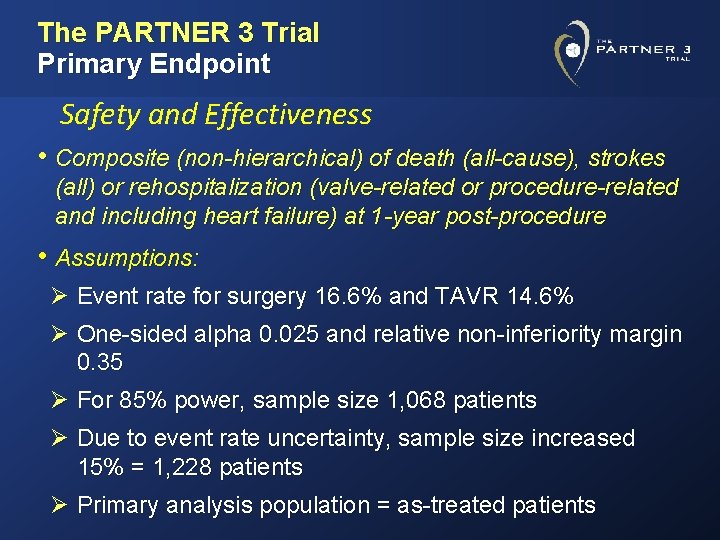
The PARTNER 3 Trial Primary Endpoint Safety and Effectiveness • Composite (non-hierarchical) of death (all-cause), strokes (all) or rehospitalization (valve-related or procedure-related and including heart failure) at 1 -year post-procedure • Assumptions: Ø Event rate for surgery 16. 6% and TAVR 14. 6% Ø One-sided alpha 0. 025 and relative non-inferiority margin 0. 35 Ø For 85% power, sample size 1, 068 patients Ø Due to event rate uncertainty, sample size increased 15% = 1, 228 patients Ø Primary analysis population = as-treated patients

The PARTNER 3 Trial Key Secondary Endpoints • Death (all-cause and CV) at 30 days and 1 -year • Strokes (with and without disability) at 30 days and 1 -year • Death (all) or strokes (all) at 30 days and 1 -year • Death (all) or strokes with disability at 30 days and 1 -year • Rehospitalization at 30 days and 1 -year • Vascular complications, bleeding, AKI, new-onset AF, new permanent pacemakers • Six-minute walk distance, QOL assessment (KCCQ scores), and NYHA Functional Class at 30 days and 1 -year • Length of stay (ICU and hospitalization) and days alive outof-hospital

The PARTNER 3 Trial Substudies and Registries (1) • CT sub-study (leaflet thickening/mobility/thrombosis) Ø 400 evaluable randomized patients (1: 1 TAVR vs. surgery) from ~20 centers with required time-resolved 3 D-MDCT imaging capabilities Ø Studies at 1 month and 1 year Ø Exclude patients with oral AC therapy • Actigraphy/QOL sub-study (cw KCCQ and 6 MWT) Ø 200 -400 evaluable randomized patients (1: 1 TAVR vs. surgery) from ~20 centers Ø Wearable actigraphy monitors at baseline and for 4 -8 weeks post-procedure

The PARTNER 3 Trial Substudies and Registries (2) • Alternative access registry Ø 100 low-risk patients with unfavorable TF access Ø Treated by TA, direct Ao, or other access routes (e. g. subclavian, carotid) • Bicuspid AS registry Ø 100 patients with severe AS and bicuspid AV disease from ~25 centers Ø TF only, “favorable” anatomy for TAVR, all risk categories • Sapien 3 Aortic and Mitral Valve-in-Valve registry Ø 150 patients; 75 aortic and 75 mitral with bioprosthetic valve failure from ~25 centers (all risk categories)

The PARTNER 3 Trial Special Considerations • Up to 60 study sites (U. S. and Canada) • 10 -year FU for all randomized patients (valve durability echocardiography assessments) • Young age not an exclusion criteria • Careful neurology evaluations to ascertain stroke endpoints • In patients with concomitant CAD, revascularization based on Heart Team assessment (within protocol guidelines) • CTAs in all patients; optimal valve sizing for both TAVR and surgery patients • Case review calls to adjudicate clinical and anatomic entry criteria

The PARTNER 3 Trial Summary • “Real World” low-risk AS study population – few exclusions • Rigorous trial design with meaningful 1 ry endpoint and numerous important 2 ry endpoints (including QOL and health economics) – VARC II and III definitions • Politically more challenging (surgeons “sweet spot”), so can’t predict enrollment cadence • 10 -year FU to address valve durability issues • Important substudies and registries – serial CTAs (valve leaflet thickening/motion), actigraphy wearables (new QOL methods), bicuspid valve disease, and aortic/mitral Vi. V