Stripping process design Prof Dr Marco Mazzotti Institut



- Slides: 8

Stripping process design Prof. Dr. Marco Mazzotti - Institut für Verfahrenstechnik

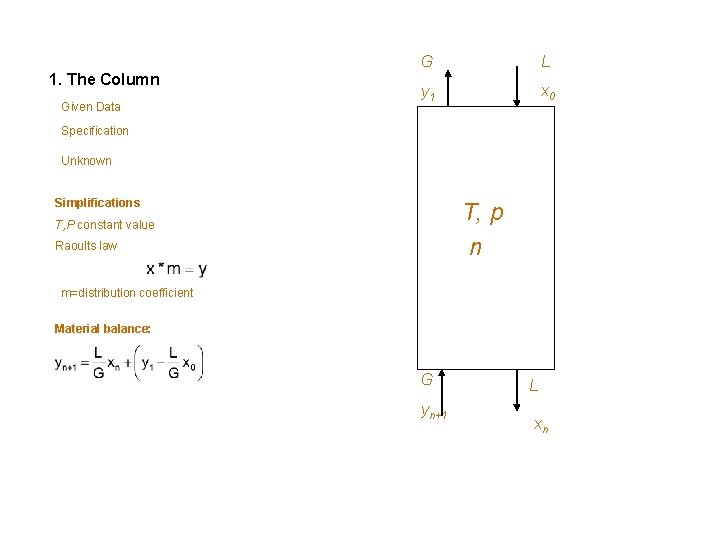
1. The Column Given Data G L y 1 x 0 Specification Unknown Simplifications T, p n T, P constant value Raoults law m=distribution coefficient Material balance: G yn+1 L xn

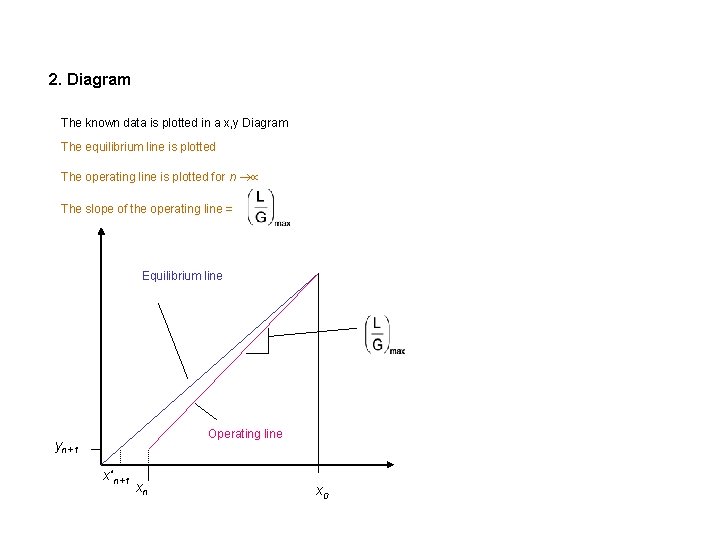
2. Diagram The known data is plotted in a x, y Diagram The equilibrium line is plotted The operating line is plotted for n The slope of the operating line = Equilibrium line Operating line yn+1 x*n+1 xn x 0

3. Dimensioning of the Column 3. 1 Calculating the necessary gas amount In the practice, the slope for the operating line is taken as 1 to 2 times the minimum slope: Good values of a are between 1. 2 and 2

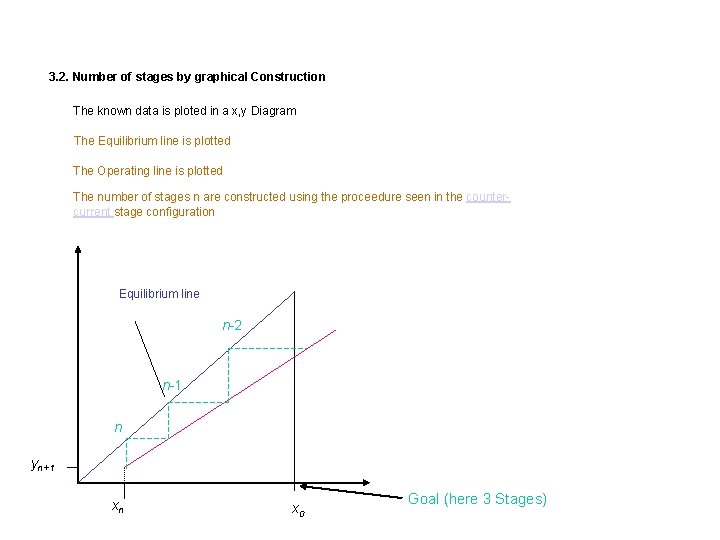
3. 2. Number of stages by graphical Construction The known data is ploted in a x, y Diagram The Equilibrium line is plotted The Operating line is plotted The number of stages n are constructed using the proceedure seen in the countercurrent stage configuration Equilibrium line n-2 n-1 n yn+1 xn x 0 Goal (here 3 Stages)

3. 3. Number of stages by Kremser Equation Fractional stripping: Stripping Factor: Kremser Equation (as shown in Counter current cascade) Solving this equation for n gives the number of stages

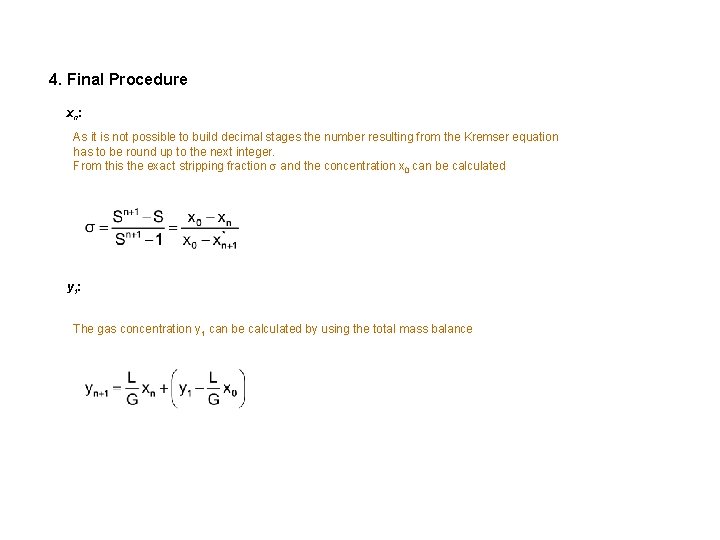
4. Final Procedure xn: As it is not possible to build decimal stages the number resulting from the Kremser equation has to be round up to the next integer. From this the exact stripping fraction and the concentration x 0 can be calculated y 1: The gas concentration y 1 can be calculated by using the total mass balance

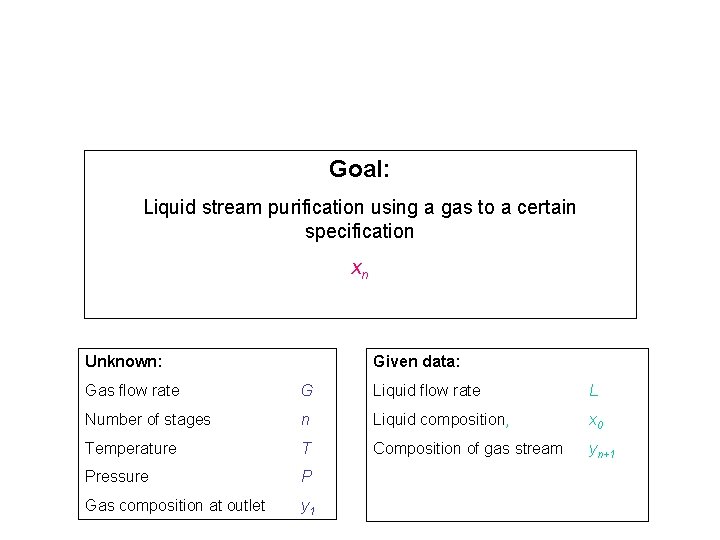
Goal: Liquid stream purification using a gas to a certain specification xn Unknown: Given data: Gas flow rate G Liquid flow rate L Number of stages n Liquid composition, x 0 Temperature T Composition of gas stream yn+1 Pressure P Gas composition at outlet y 1