Static Electricity Physics Mrs Coyle http www uwec






















- Slides: 41

Static Electricity Physics Mrs. Coyle http: //www. uwec. edu/jolhm/EH 3/Group 2/Pictures/lightning. jpg

Part I • • History Charge and its Conservation Conductors, Semiconductors, Insulators Methods of Charging

History • Electron means “amber” in Greek • Thales of Miletos 600 BC discovered properties by Greek. • He rubbed amber (mineral) with cat fur and attracted feathers.

Ben Franklin’s Kite Experiment (1740’s)

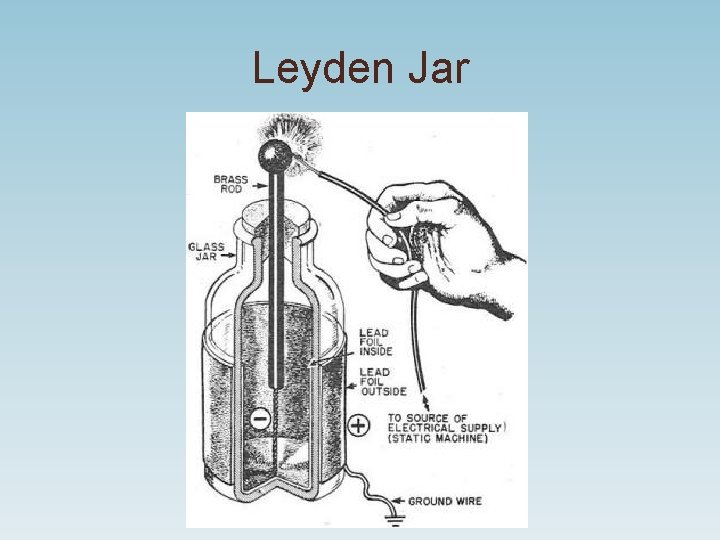
Leyden Jar

Benjamin Franklin • 1740’s lightning experiment with kite, key and Leyden jar (stores static electricity). • Franklin developed the lightning rod. • Proposed conservation of charge. • Saw electricity as a flowing fluid and called the flow direction positive.

Law of Conservation of Electrical Charge • The net charge of an isolated system remains constant.

Example: • An object of +10 C touched an identical object that was neutral. What is the charge of each object?

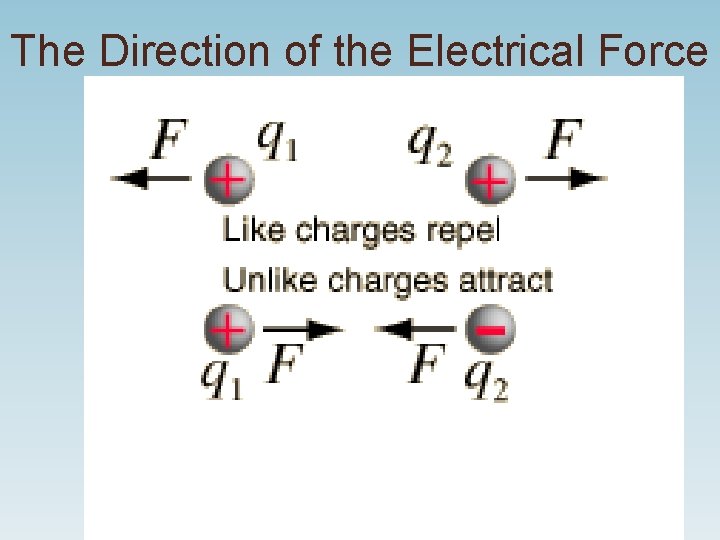
Law of Charges • Like charges repel • Opposite charges attract

J(oseph) J(ohn) Thomson (1897, England) • He discovered the electron. • He found that the mass of the electron is about 1/1800 of the mass of a hydrogen atom. • He won the Nobel Prize (1906) for his discovery of the electron.

JJ Thomson with the CRT

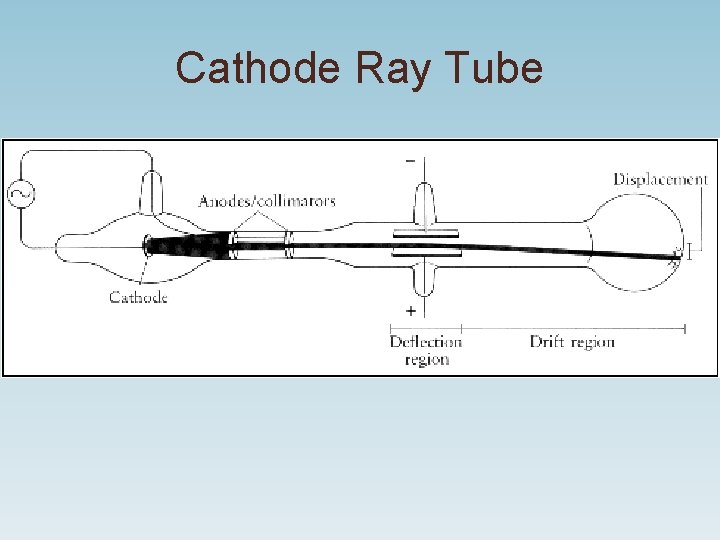
Cathode Ray Tube

Cathode Ray

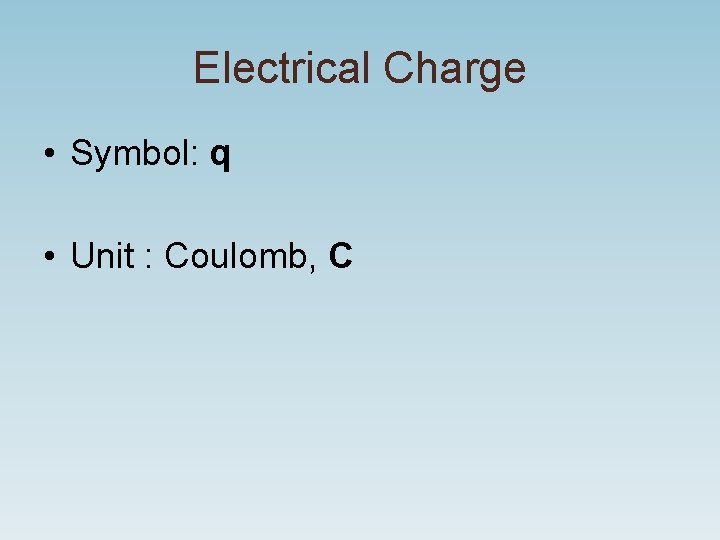
Electrical Charge • Symbol: q • Unit : Coulomb, C

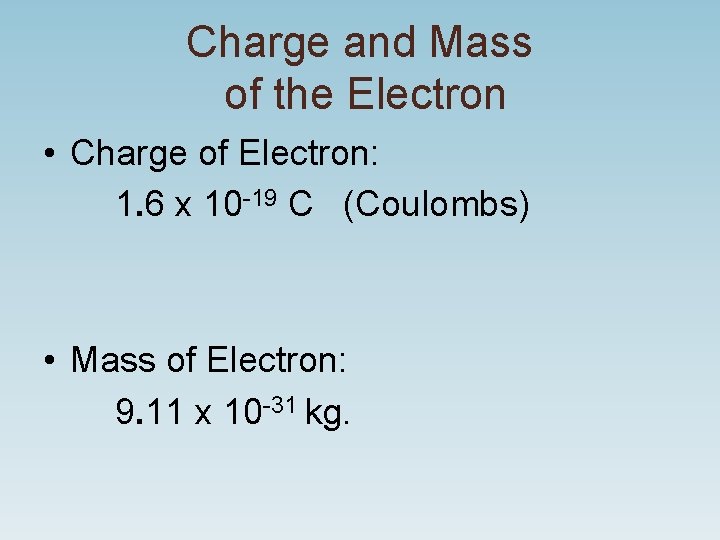
Charge and Mass of the Electron • Charge of Electron: 1. 6 x 10 -19 C (Coulombs) • Mass of Electron: 9. 11 x 10 -31 kg.


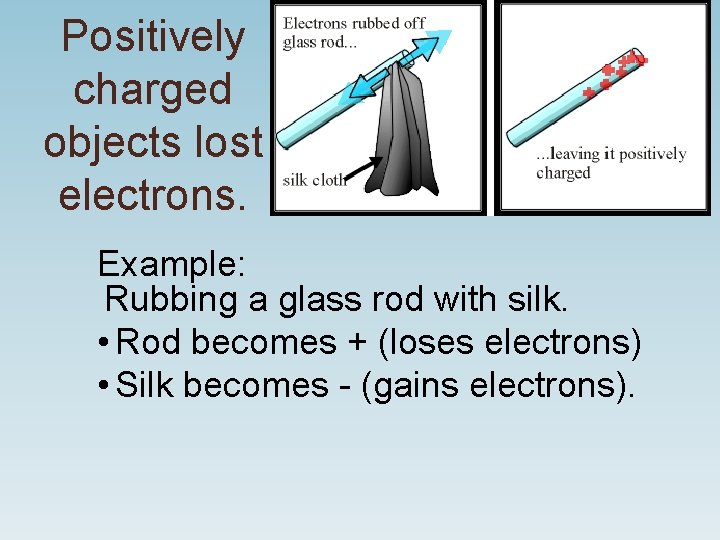
Positively charged objects lost electrons. Example: Rubbing a glass rod with silk. • Rod becomes + (loses electrons) • Silk becomes - (gains electrons).

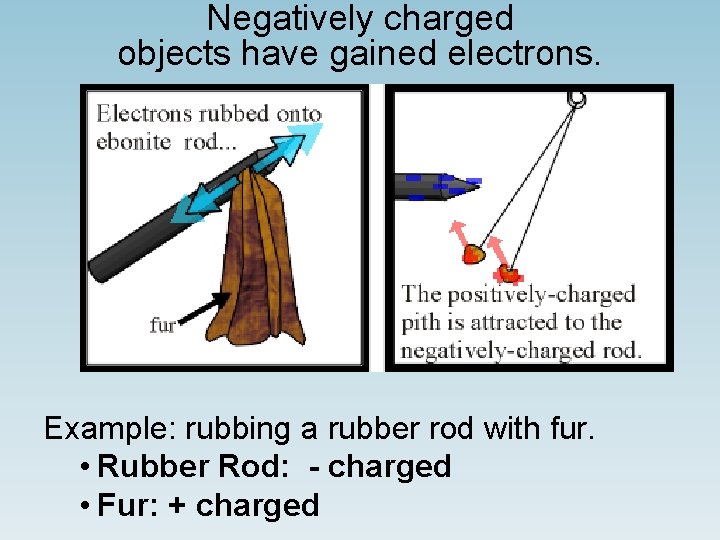
Negatively charged objects have gained electrons. Example: rubbing a rubber rod with fur. • Rubber Rod: - charged • Fur: + charged

Note • Negatively charged objects have more mass than an identical neutral object, since each extra electron has a mass of 9. 11 x 10 -31 kg.

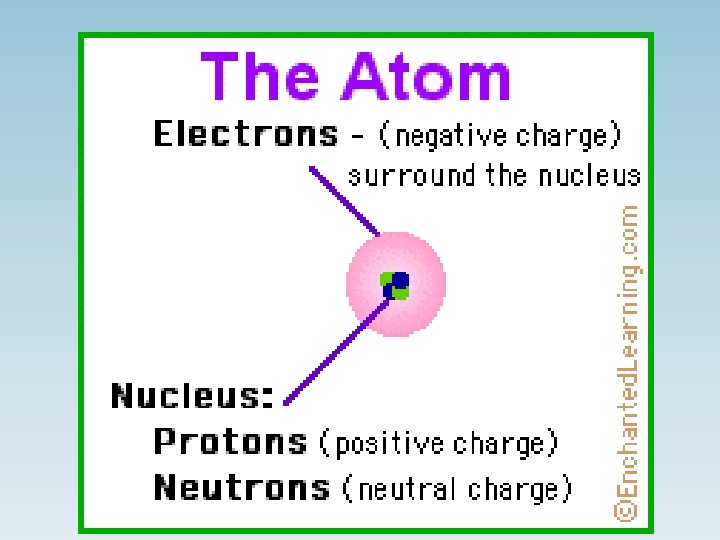
Types of Materials in terms of Electrical Conductivity • Conductors (metals) • Semiconductors (germanium, silicon) • Insulators (wood, glass, rubber)

Electrostatic Charging Methods • Friction • Conduction • Induction

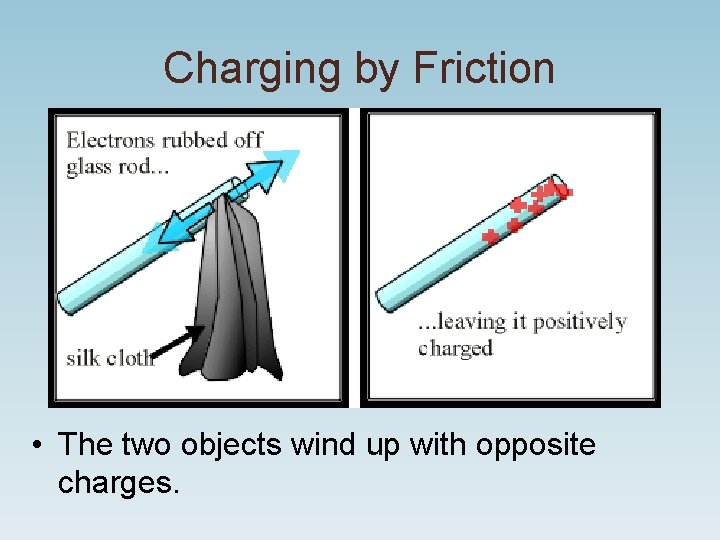
Charging by Friction • The two objects wind up with opposite charges.

Triboelectric Series + Fur (rabbit) Glass Wool Fur (cat) Lead Silk Human skin, Aluminum Cotton Wood Amber Nickel, Copper, Brass, Gold Rubber Sulfur - Celluloid

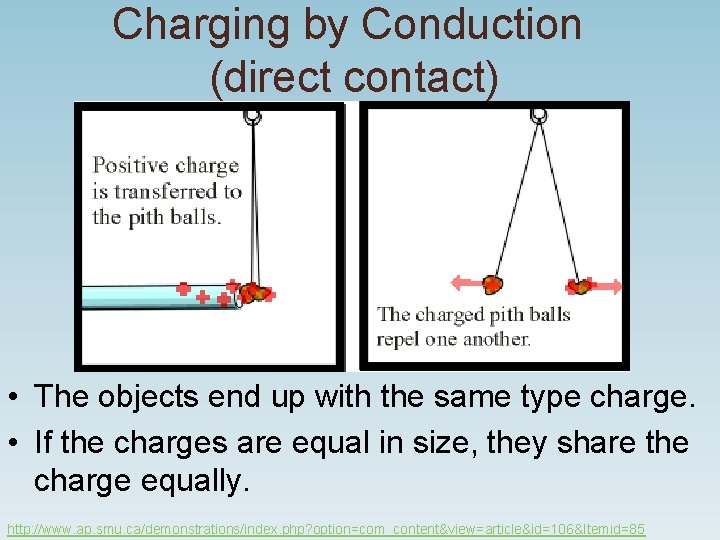
Charging by Conduction (direct contact) • The objects end up with the same type charge. • If the charges are equal in size, they share the charge equally. http: //www. ap. smu. ca/demonstrations/index. php? option=com_content&view=article&id=106&Itemid=85

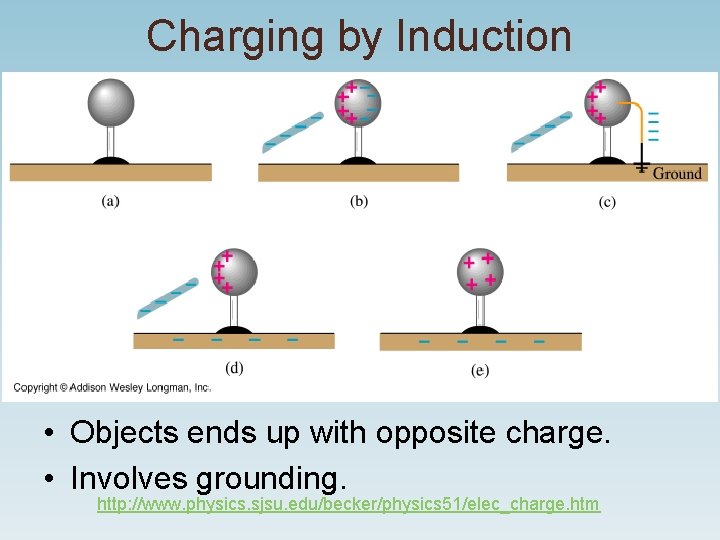
Charging by Induction • Objects ends up with opposite charge. • Involves grounding. http: //www. physics. sjsu. edu/becker/physics 51/elec_charge. htm

Electroscopes are used to test the charge of an object.

When a charged object is brought near the electroscope, its leaves spread apart. http: //upload. wikimedia. org/wikipedia/commons/e/ec/Electroscope_showing_induction. png

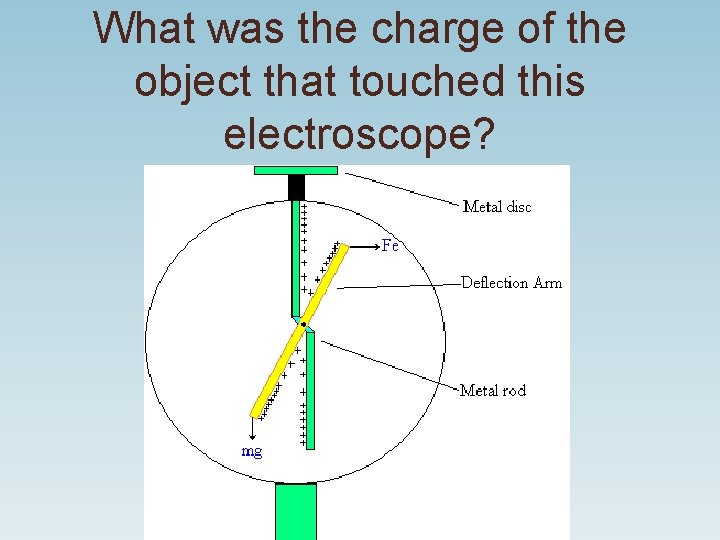
When a charged object touches an electroscope, the electroscope is now charged.

What was the charge of the object that touched this electroscope?

Polarization http: //www. csiro. au/helix/sciencemail/activities/Water. Bend. html

Part II • Coulomb’s Law

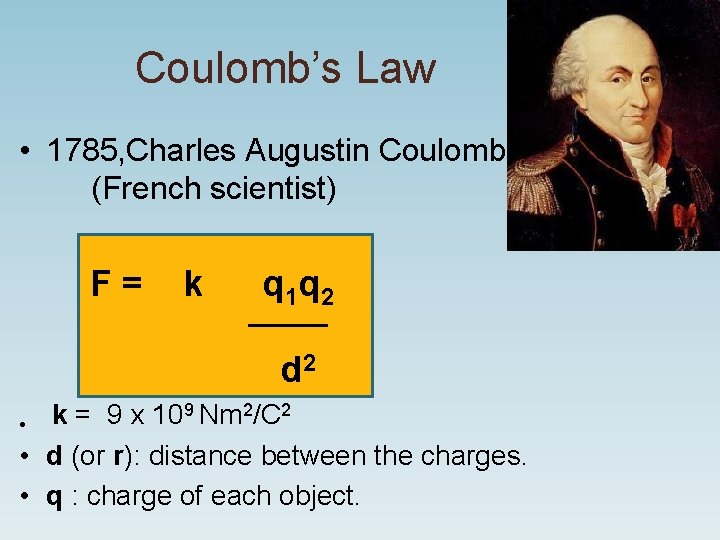
Coulomb’s Law • 1785, Charles Augustin Coulomb (French scientist) F = k q 1 q 2 ______ d 2 9 Nm 2/C 2 k = 9 x 10 • • d (or r): distance between the charges. • q : charge of each object.

The Direction of the Electrical Force

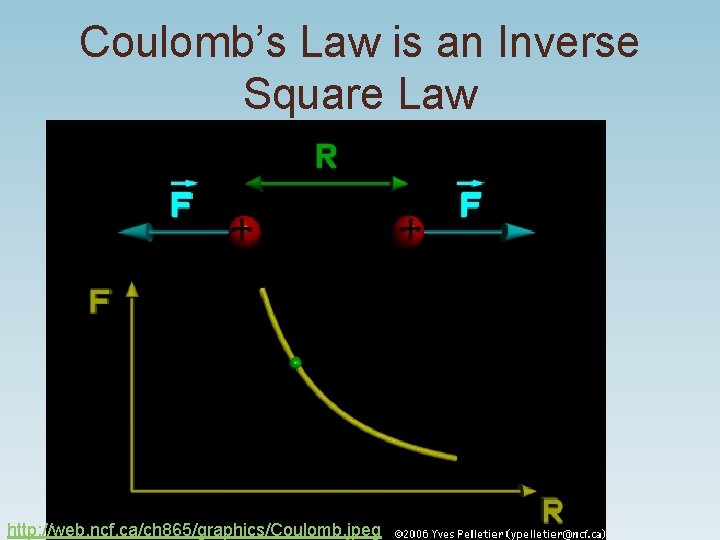
Coulomb’s Law is an Inverse Square Law http: //web. ncf. ca/ch 865/graphics/Coulomb. jpeg

The electrical force is one of the four fundamental forces.

Comparison with Gravitational Force • What are 3 differences between the electrical force and the gravitational force?

Comparison with Gravitational Force • What are 3 differences between the electrical force and the gravitational force?

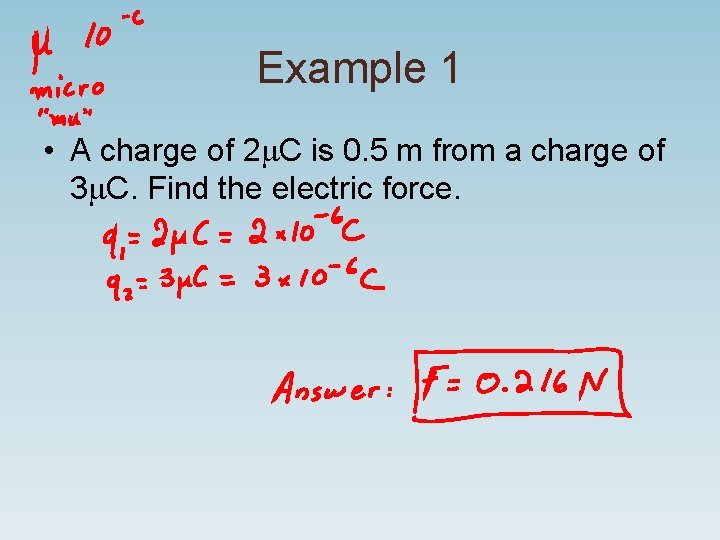
Example 1 • A charge of 2 m. C is 0. 5 m from a charge of 3 m. C. Find the electric force.

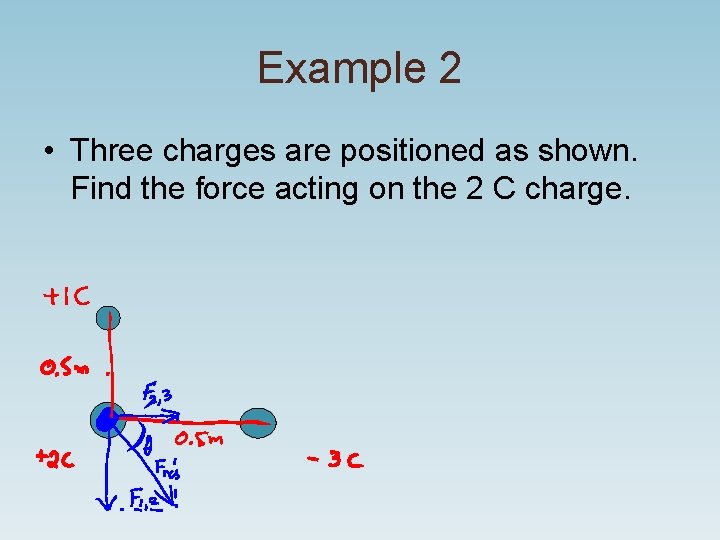
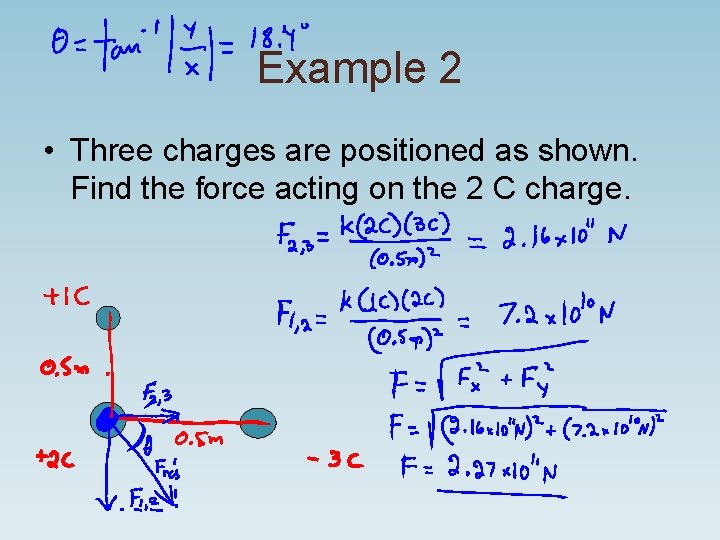
Example 2 • Three charges are positioned as shown. Find the force acting on the 2 C charge.

Example 2 • Three charges are positioned as shown. Find the force acting on the 2 C charge.

Example 3 Two equal charges are located 1 m from each other. The force acting between them is 2 N. How many Coulombs is each charge? Answer: 15μC