Somatic cell Nuclear Transfer In genetics and developmental











- Slides: 17

Somatic cell Nuclear Transfer • In genetics and developmental biology, somatic-cell nuclear transfer (SCNT) is a laboratory technique for creating a clone embryo with a donor nucleus • It can be used in embryonic stem cell research, or, potentially, in regenerative medicine where it is sometimes referred to as "therapeutic cloning". • It can also be used as the first step in the process of reproductive cloning.

• First explored by Hans Spemann( 1920) to conduct genetics research, nuclear transfer is the technique currently used in the cloning of adult animals. • A technique known as twinning exists, but can only be used before an organism’s cells differentiate. • N T requires two cells, a donor cell and an oocyte, or egg cell. • Research has proven that the egg cell works best if it is unfertilized, because it is more likely to accept the donor nucleus as its own. • The egg cell must be enucleated. • The donor cell is then forced into the Gap Zero, or G 0 cell stage, a dormant phase, in different ways depending on the technique.

• This dormant phase causes the cell to shut down but not die. • In this state, the nucleus is ready to be accepted by the egg cell. The donor cell’s nucleus is then placed inside the egg cell, either through cell fusion or transplantion. • The egg cell is then prompted to begin forming an embryo. When this happens, the embryo is then transplanted into a surrogate mother. • Each group of researchers has its own specific technique. • The best known is -- Roslin technique, and the most effective and most recently developed is the Honolulu technique.


Limitations • Stresses placed on both the egg cell and the introduced nucleus are enormous, leading to a high loss in resulting cells. For eg, Dolly the sheep was born after 277 eggs used for SCNT, which created 29 viable embryos. Only 3 of these embryos survived until birth, and only 1 survived to adulthood. • As the procedure currently cannot be automated, but has to be performed manually under a microscope, it is very resource intensive.

Biochemistry involved in reprogramming the differentiated somatic cell nucleus and activating the recipient egg is also far from understood. In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's mitochondria that contain their own mitochondrial are left behind. Resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus

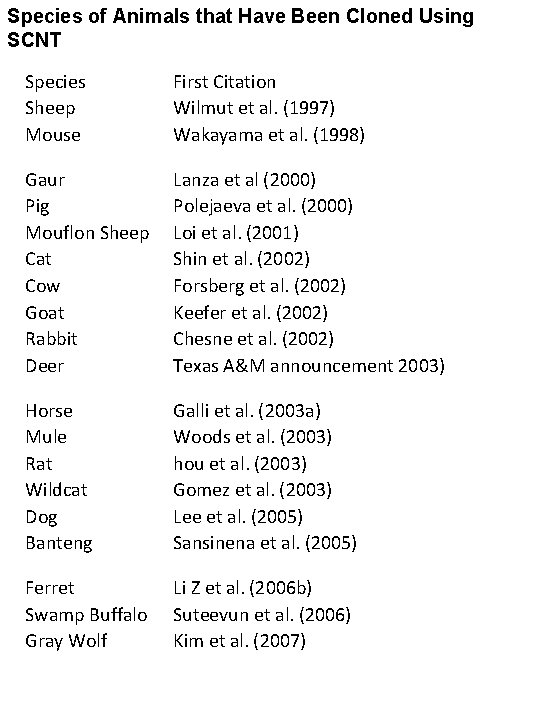
Species of Animals that Have Been Cloned Using SCNT Species Sheep Mouse First Citation Wilmut et al. (1997) Wakayama et al. (1998) Gaur Pig Mouflon Sheep Cat Cow Goat Rabbit Deer Lanza et al (2000) Polejaeva et al. (2000) Loi et al. (2001) Shin et al. (2002) Forsberg et al. (2002) Keefer et al. (2002) Chesne et al. (2002) Texas A&M announcement 2003) Horse Mule Rat Wildcat Dog Banteng Galli et al. (2003 a) Woods et al. (2003) hou et al. (2003) Gomez et al. (2003) Lee et al. (2005) Sansinena et al. (2005) Ferret Swamp Buffalo Gray Wolf Li Z et al. (2006 b) Suteevun et al. (2006) Kim et al. (2007)

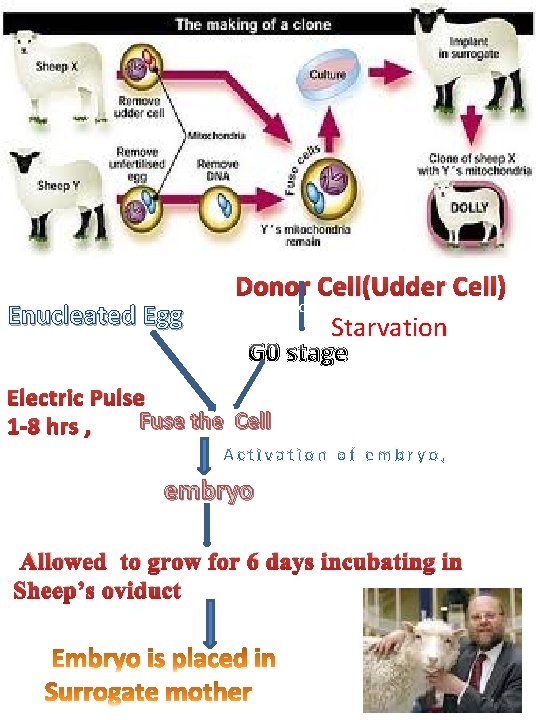
Roslin technique • • • ( Ian Wilmut and Keith Cambell) Previously, it was not known if an adult nucleus was still able to produce a completely new animal. Genetic damage and the simple deactivation of genes in cells were both considered possibly irreversible. Cell synchronization- forced into the Gap Zero, or G 0 cell stage, or the dormant cell stage. Udder cell (the donor cell) was selected from a Finn Dorset sheep to provide the genetic information for the clone. allowed the cell to divide and form a culture in vitro, or outside of an animal. This produced multiple copies of the same nucleus.

Enucleated Egg Donor Cell(Udder Cell) c Starvation G 0 stage Electric Pulse Fuse the Cell 1 -8 hrs , embryo Allowed to grow for 6 days incubating in Sheep’s oviduct

• 1998 - University of Hawaii- Teruhiko wakayama and Ryuzo Yanagimachi. • Mice had long been held to be one of the most difficult mammals to clone due to the fact that almost immediately after a mouse egg is fertilized, it begins dividing. • Sheep were used in the Roslin technique because their eggs wait several hours before dividing, possibly giving the egg time to reprogram its new nucleus. • Even without this luxury, Wakayama and Yanagimachi were able to clone with a much higher success rate (three clones out of every one-hundred attempts) than Ian Wilmut (one in 277).

Wakayama approached the problem of synchronizing cell cycles differently than Wilmut used udder cells, which had to be forced into the G 0 stage. Wakayama- 3 types of cells Sertoli Cells brain cells, Cumulus Cells Sertoli and brain cells both remain in the G 0 state naturally and cumulus cells are almost always in either the G 0 or G 1 state.

Unfertilized mouse egg cells being enucleateddonor nuclei inserted into them. (Micromanipulation) The donor nuclei were taken from cells within minutes of the each cell’s extraction from a mouse. Unlike the process used to create Dolly, no in vitro or outside of an animal, culturing was done on the cells. After one hour, the cells had accepted the new nucleus

• After an additional five hours, the egg cell was then placed in a chemical culture to jumpstart the cell’s growth, just as fertilization does in nature. • In the culture was a substance (cytochalasin B) which stopped the formation of a polar body, a second cell which normally forms before fertilization. • The polar body would take half of the genes of the cell, preparing the other cell to receive genes from sperm.

After being jumpstarted, the cells develop into embryos. transplanted into surrogate mothers and carried to term. Most successful of the cells for the process were cumulus cells, so research was concentrated on cells of that type.

After proving that the technique was viable, Wakayama also made clones of clones and allowed the original clones to give birth normally to prove that they had full reproductive functions. At the time he released his results, Wakayama had created fifty clones.

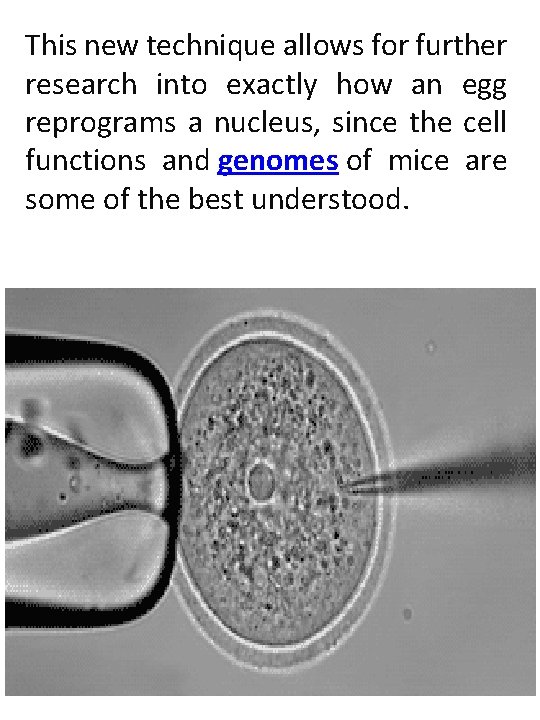
This new technique allows for further research into exactly how an egg reprograms a nucleus, since the cell functions and genomes of mice are some of the best understood.

Animals that have been Cloned • • Sheep Goats Cows Mice Pigs Cats Rabbits Gaur