Solids Solids are formed when temperatures are low








- Slides: 10

Solids • Solids are formed when temperatures are low enough to prevent molecules from moving around their neighbors • Molecules or atoms in solids vibrate/oscillate around a fixed position – All the atoms in a solid essentially move in unison

Types of Solids • Two major classifications: Crystalline and Amorphous 1. Crystalline Solids: All atoms, ions or molecules in the solid lie in an orderly array. Long range order exists within a crystalline solid 2. Amorphous Solids: The atoms, ions or molecules in an amorphous solid lie in a seemingly random arrangement. • • Amorphous solids look like we took a snapshot of a liquid Glass is an amorphous solid

Crystalline Solids 1. Molecular Solids: Assemblies of discrete molecules held together by intermolecular forces (eg: Quartz) 2. Network Solids: Atoms are covalently bonded to their neighbors throughout the extent of the solid (eg: Diamonds) 3. Metallic Solids: Metal cations held together by a sea of electrons 4. Ionic Solids: Built from the mutual attraction of anions and cations


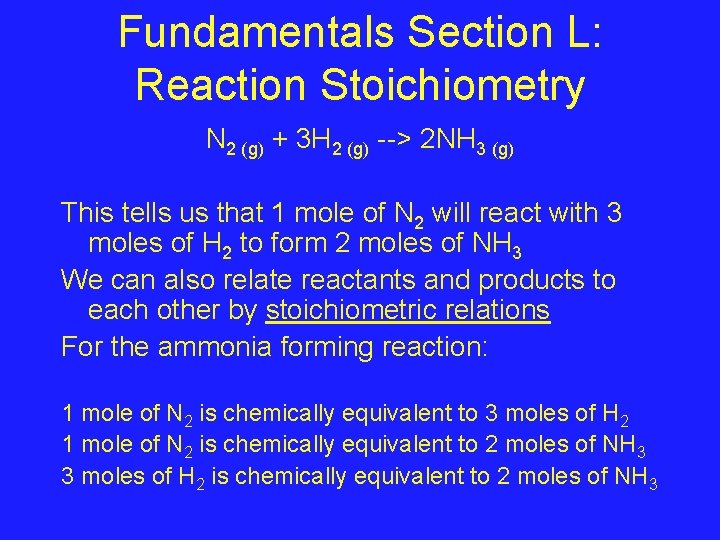
Fundamentals Section L: Reaction Stoichiometry N 2 (g) + 3 H 2 (g) --> 2 NH 3 (g) This tells us that 1 mole of N 2 will react with 3 moles of H 2 to form 2 moles of NH 3 We can also relate reactants and products to each other by stoichiometric relations For the ammonia forming reaction: 1 mole of N 2 is chemically equivalent to 3 moles of H 2 1 mole of N 2 is chemically equivalent to 2 moles of NH 3 3 moles of H 2 is chemically equivalent to 2 moles of NH 3

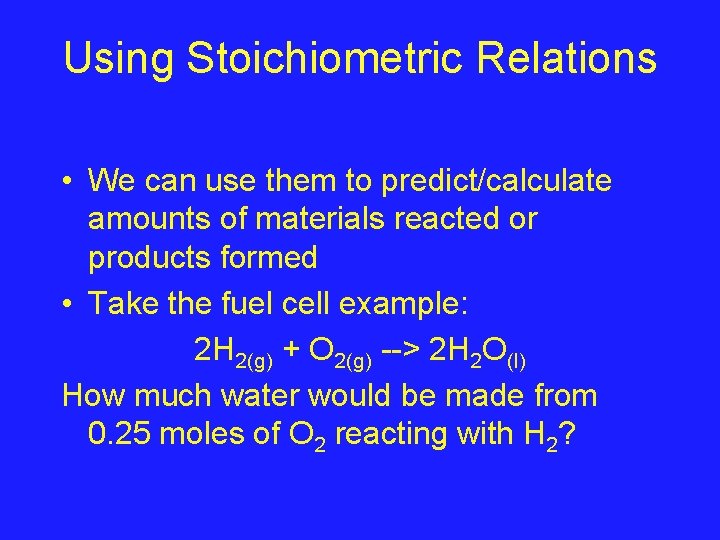
Using Stoichiometric Relations • We can use them to predict/calculate amounts of materials reacted or products formed • Take the fuel cell example: 2 H 2(g) + O 2(g) --> 2 H 2 O(l) How much water would be made from 0. 25 moles of O 2 reacting with H 2?

• Looking at the chemical equation, we can see that: 1 mole of O 2 is chemically equivalent to 2 moles of H 2 O We can set up a conversion factor based upon this relationship for use in calculations: Now we can use the stoichiometric relationship:


Calculate the mass of potassium metal needed to react with 0. 450 g of hydrogen gas to produce solid potassium hydride. Step 1: Balanced chemical reaction Step 2: Take what you’re given and convert it to moles Step 3: Use the stoichiometric coefficients to convert moles of one substance to moles of another Step 4: Convert Moles to Mass

Potassium superoxide, KO 2, is utilized in a closed system breathing apparatus to remove carbon dioxide and water from exhaled air. The removal of water generates oxygen for breathing by the reaction: 4 KO 2(s) + 2 H 2 O(l) --> 3 O 2(g) + 4 KOH(s) Potassium hydroxide removes carbon dioxide from the apparatus by the reaction: KOH(s) +CO 2(g) --> KHCO 3(g) • What mass of potassium superoxide generates 115 g of O 2? • What mass of CO 2 can be removed from the apparatus by 75. 0 g of KO 2?