Similac Special Care and Elecare cause neonatal gut











- Slides: 20

Similac Special Care and Elecare cause neonatal gut injury in mice Karishma Rao, Heather Menden, Wei Yu, Inamul Haque, Susana-Chavez Bueno, Alain Cuna, Shahid Umar, Venkatesh Sampath University of Missouri- Kansas City, MO Children’s Mercy Kansas City, MO University of Kansas Medical Center, Kansas City, KS

DISCLOSURES No financial disclosures

BACKGROUND: INCIDENCE • About 10% of all births in the United States are premature i. e. , born before 37 weeks of gestation • Necrotizing Enterocolitis (NEC) affects 5 -10% of all premature infants <1500 grams • NEC is a leading cause of overall infant mortality 15 -40% of infants with NEC can die from this disease

BACKGROUND: ETIOPATHOGENESIS • Risk factors for NEC include: • Prematurity • Genetics • Formula feeding vs mother’s own milk (nearly 2 X increased risk) • Altered gut microbiome • Formula is used in the NICU for preterm infants when: • Mother’s own milk is unavailable • Donor milk is unavailable • Poor growth

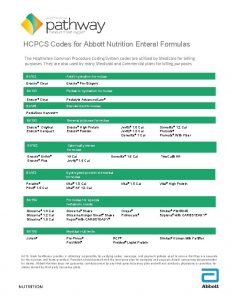
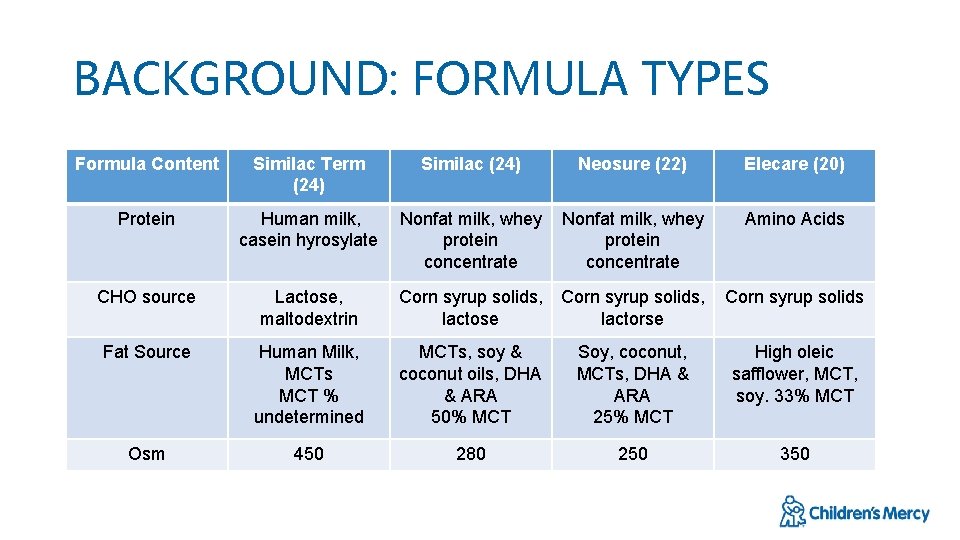
BACKGROUND: FORMULA TYPES Formula Content Similac Term (24) Similac (24) Neosure (22) Elecare (20) Protein Human milk, casein hyrosylate Nonfat milk, whey protein concentrate Amino Acids CHO source Lactose, maltodextrin Corn syrup solids, lactose Corn syrup solids, lactorse Corn syrup solids Fat Source Human Milk, MCTs MCT % undetermined MCTs, soy & coconut oils, DHA & ARA 50% MCT Soy, coconut, MCTs, DHA & ARA 25% MCT High oleic safflower, MCT, soy. 33% MCT Osm 450 280 250 350

GAP IN KNOWLEDGE • There are limited studies on the effects of preterm formula on the incidence and severity of NEC • There are limited animal studies on the effects of different preterm formulae on the developing, immature gut and microbiome

HYPOTHESIS • Different preterm formulae will vary in their ability to cause intestinal injury and alter the microbiome in the developing gut

AIMS • To quantify the effects of different preterm formulae: Elecare, Similac and Neosure on gut inflammation and severity of intestinal injury in neonatal mice • To demonstrate the protective effects of probiotic treatment on formula-fed induced gut injury • To determine the effects of different human preterm formulae on gut dysbiosis in mice

METHODS • C 57 BL/6 mice are used • They are allowed to breed naturally and spontaneously birth at term • Pups in the control group stayed with their dams and were allowed to be dam fed ad lib • All pups in the formula fed groups were fed fortified formula (26 Kcal/oz)

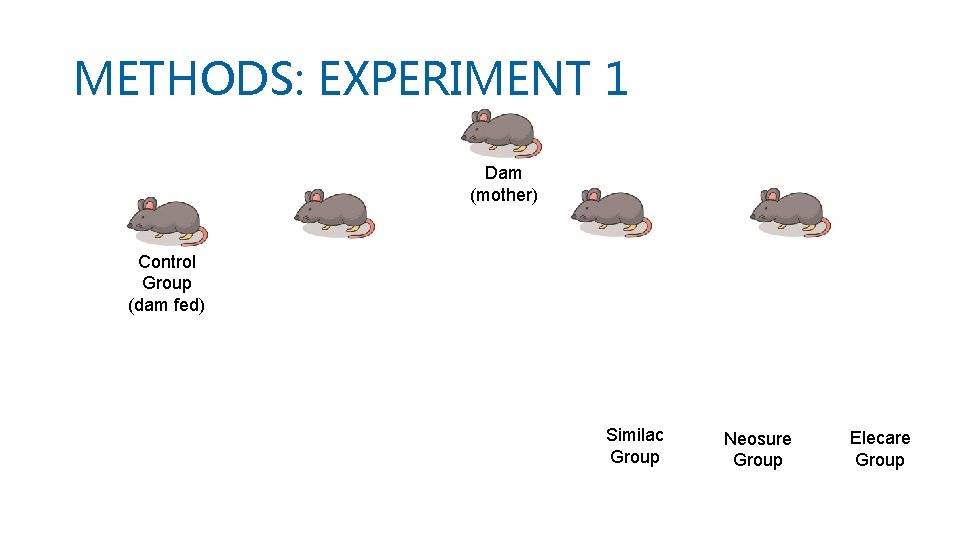
METHODS: EXPERIMENT 1 • Litters of mice were dam fed by mother till day of life 7, they were then separated from mothers and randomly assigned to: control group and preterm formula feeding groups • Pups in the preterm formula group were randomly divided into 3 groups based on formula type and were gavage fed fortified formula: Similac (S), Neosure (N), Elecare (E) • The preterm formula fed mice were gavage fed five times a day on day of life 8, 9, 10

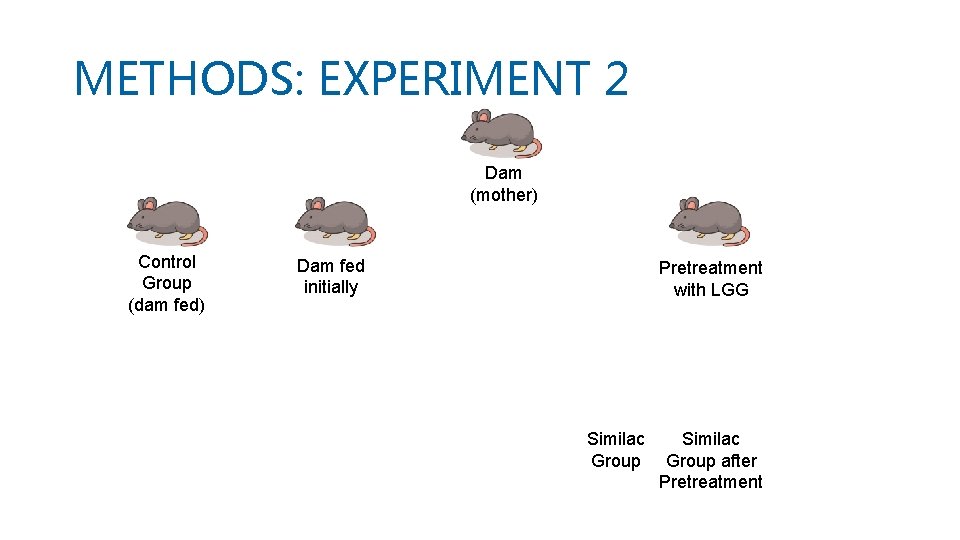
METHODS: EXPERIMENT 2 • Litters of mice were dam fed till day of life 7, they were then separated from mothers and randomly assigned to: control group and preterm formula feeding groups • Pups in the preterm formula feeding group were pre-treated with Lactobacillus rhamnosus (LGG) from day 5 to 7, followed by formula feeding of S or E on day 8, 9, 10 • All mice pups were killed on day of life 11 by intraperitoneal injection of pentobarbital and exsanguination

METHODS: EXPERIMENT 1 Dam (mother) Control Group (dam fed) Similac Group Neosure Group Elecare Group

METHODS: EXPERIMENT 2 Dam (mother) Control Group (dam fed) Dam fed initially Pretreatment with LGG Similac Group after Pretreatment

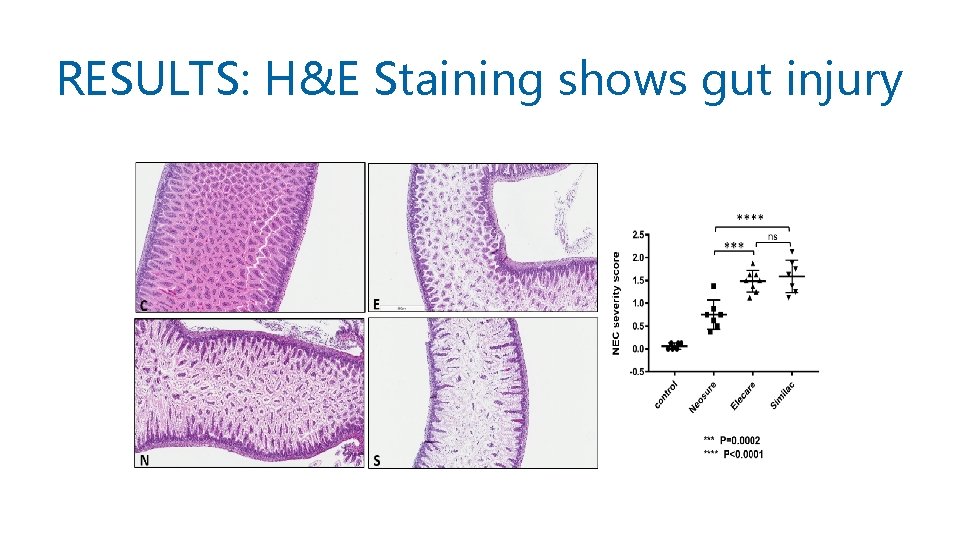
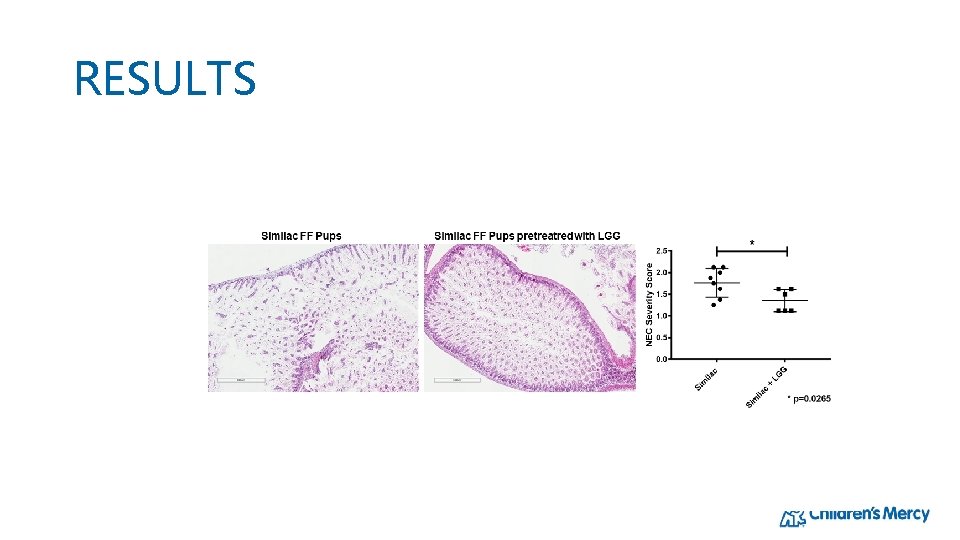
RESULTS: H&E Staining shows gut injury

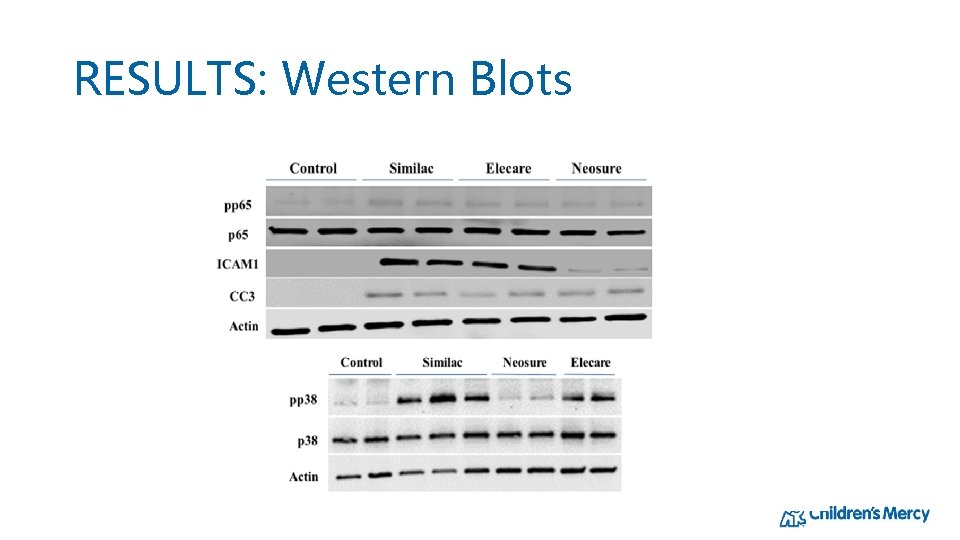
RESULTS: Western Blots

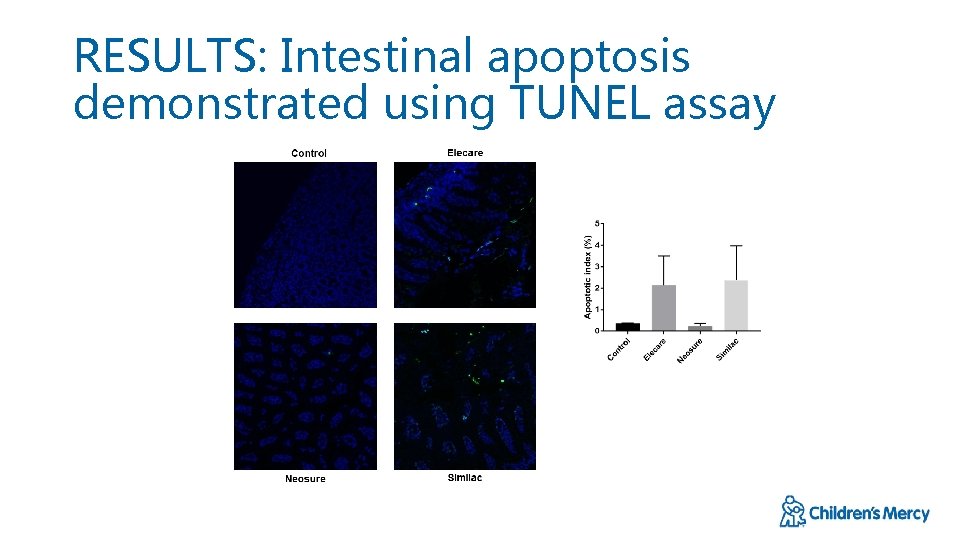
RESULTS: Intestinal apoptosis demonstrated using TUNEL assay

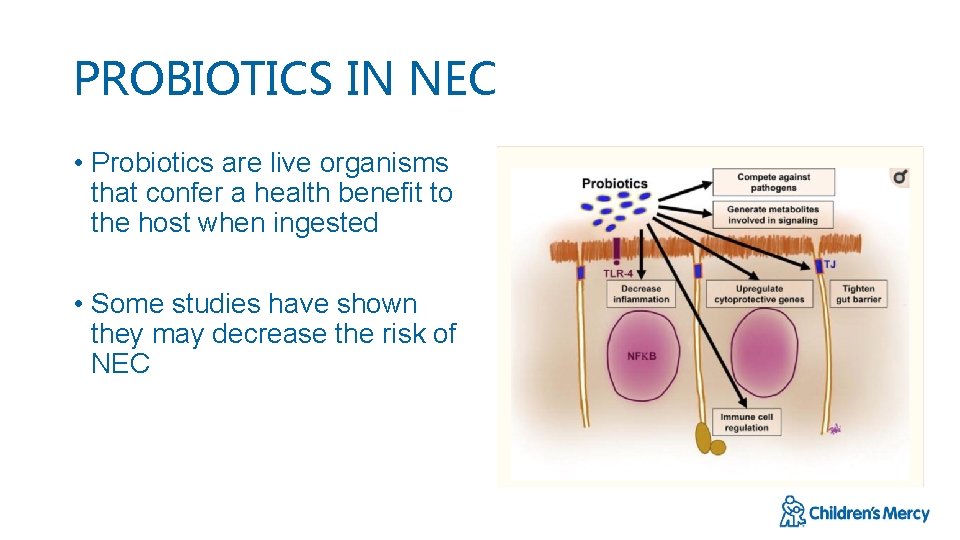
PROBIOTICS IN NEC • Probiotics are live organisms that confer a health benefit to the host when ingested • Some studies have shown they may decrease the risk of NEC

RESULTS

NEXT STEPS • Completing PCRs and TUNELs (ongoing) on formula feeding and LGG groups • Microbiome analysis sent for sequencing. Results awaited in April, COVID delay - May

 Wic similac total comfort
Wic similac total comfort Glucerna cpt code
Glucerna cpt code Formule elecare
Formule elecare Levels of nursing care primary secondary tertiary
Levels of nursing care primary secondary tertiary Ter death certificate
Ter death certificate Ultimate cause of behavior
Ultimate cause of behavior Proximate vs ultimate causation
Proximate vs ultimate causation Imprinting example
Imprinting example Gut and tripe room
Gut and tripe room Entrepreneurial mind frame heart flame and gut game meaning
Entrepreneurial mind frame heart flame and gut game meaning Hallo wie geht es dir mir geht es gut
Hallo wie geht es dir mir geht es gut Zustandsformen wasser arbeitsblatt
Zustandsformen wasser arbeitsblatt Ruh oder ruhe dich aus
Ruh oder ruhe dich aus Leo galland leaky gut
Leo galland leaky gut Blind gut disadvantages
Blind gut disadvantages Gut g?tesiegel
Gut g?tesiegel Ventral mesogastrium
Ventral mesogastrium Ventral mesentery derivatives
Ventral mesentery derivatives Hallo wie geht es dir lied
Hallo wie geht es dir lied Three disadvantages of having a blind gut in porifera
Three disadvantages of having a blind gut in porifera Lieber schlecht gefahren als gut gelaufen
Lieber schlecht gefahren als gut gelaufen