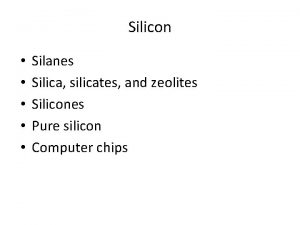
SILANES H Si H Hydrides of Silicon are
- Slides: 5

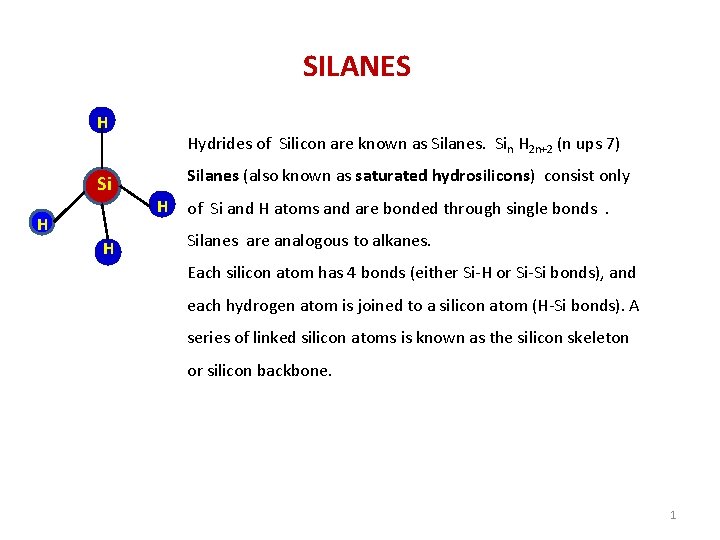
SILANES H Si H Hydrides of Silicon are known as Silanes. Sin H 2 n+2 (n ups 7) Silanes (also known as saturated hydrosilicons) consist only H of Si and H atoms and are bonded through single bonds. Silanes are analogous to alkanes. Each silicon atom has 4 bonds (either Si-H or Si-Si bonds), and each hydrogen atom is joined to a silicon atom (H-Si bonds). A series of linked silicon atoms is known as the silicon skeleton or silicon backbone. 1

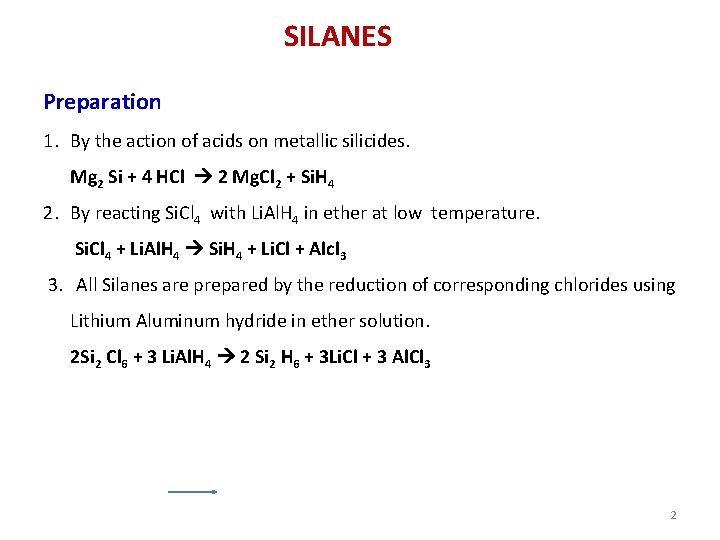
SILANES Preparation 1. By the action of acids on metallic silicides. Mg 2 Si + 4 HCl 2 Mg. Cl 2 + Si. H 4 2. By reacting Si. Cl 4 with Li. Al. H 4 in ether at low temperature. Si. Cl 4 + Li. Al. H 4 Si. H 4 + Li. Cl + Alcl 3 3. All Silanes are prepared by the reduction of corresponding chlorides using Lithium Aluminum hydride in ether solution. 2 Si 2 Cl 6 + 3 Li. Al. H 4 2 Si 2 H 6 + 3 Li. Cl + 3 Al. Cl 3 2

SILANES Properties 1. All the silanes are colourless volatile covalent compounds. 2. The first two silanes are gases while other are liquids 3. Action of alkali solution : Silanes are soluble in strong alkali solution in presence of air and evolve H 2 2 Si. H 4 + 4 Na. OH + O 2 2 Na 2 Si. O 3 + 6 H 2 4. Action of HCl & HBr Si. H 4 when treated with HCl or HBr at 1000 C in presence of a catalyst Al 2 Cl 3 results in the substitution of H-atom by Cl/Br atom. Al 2 Cl 3 Si. H 4 + 4 HCl Si. H 3 Cl + H 2 3

SILANES 5. Action of heat : when heated to v. high temperatures, silanes break into its elements 670 k 470 k Si. H 4 Si + 2 H 2 Si 2 H 6 2 Si + 3 H 2 ∆ ∆ 6. Action of salts : Ag is pptd when Si. H 4 reacts with Agcl in heated flow reactor 200 0 C Si. H 4 + 2 Ag. Cl Si. H 3 Cl + HCl + 2 Ag Silanes have structure similar to alkanes i. e. to those of the corresponding saturated hydrocarbons. Difference between alkanes and silanes Hydrocarbons Silanes 1. Its number of carbon atoms in a chain can have any value 2. CH 4 does not get hydrolysed. 3. maximum covalency of carbon is four which is satisfied in CH 4 hence it resists the attack of e- donating molecules 1. Maximum number of silicon atom is seven. 2. Si. H 4 gets hydrolysed. 3. Mximum covalency of Si is six, but only 4 bonds are present, so Si accepts ions pair of e- s from e- donating molecules 4

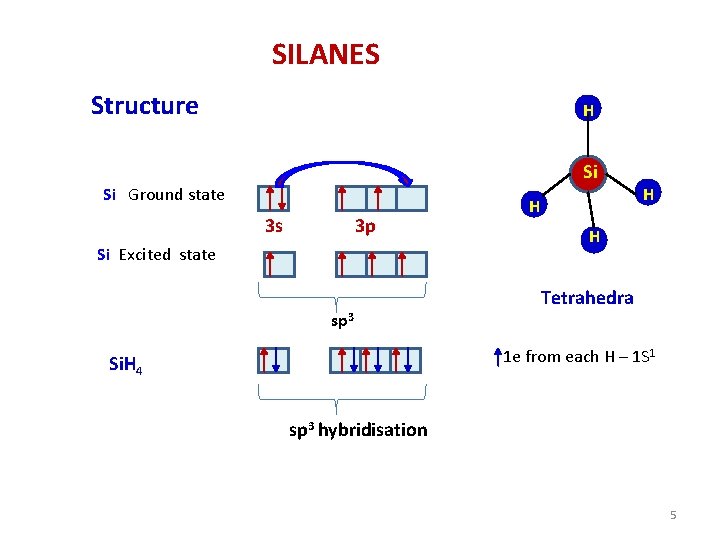
SILANES Structure H H H Si Si Ground state 3 s 3 p Si Excited state sp 3 H H H Tetrahedra 1 e from each H – 1 S 1 Si. H 4 sp 3 hybridisation 5