Sept 15 3 pts 1 3 pts 2




- Slides: 9

Sept 15: 3 pts 1. 3 pts 2. 3 pts 3. 3 pts 4. 3 pts 5. 5 pts 6. Why is it important to make careful observations during a lab? Give three examples of physical properties of the substance in the cup. What do you do if there are choking fumes in the lab Which branch of chemistry involves hydrocarbons? Did the oil adhere (stick) to the sand? If detergent could be used to break up the oil, would this be an effective and safe way to clean up the oil? Why (not)? 1. So you can interpret your data accurately. 2. hard, jagged edges, silvery – whitish, metallic. Not very dense for a metal. Evacuate. (Get out of the room, closing the door behind you. ) Organic chemistry Yes, there was less oil on the surface after the sand was added. In some ways it would be effective, it might lessen the impact of the oil slick by reducing the number of animals which drown, but it may cause other problems, …. 3. 4. 5. 6.

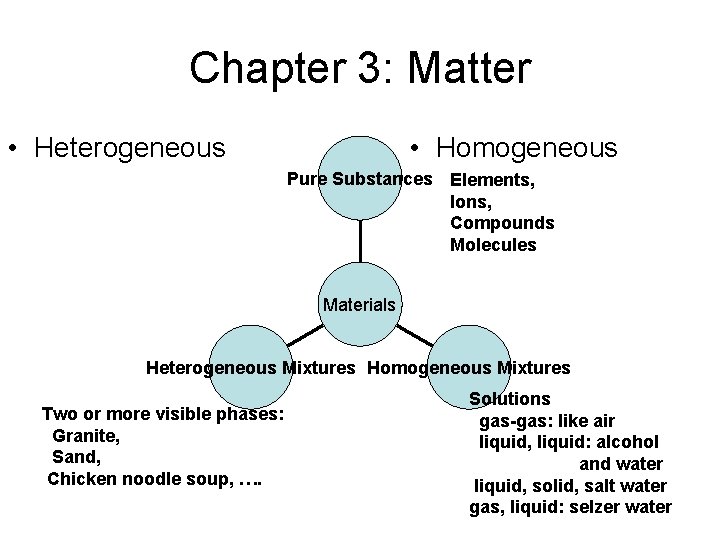
Chapter 3: Matter • Heterogeneous • Homogeneous Pure Substances Elements, Ions, Compounds Molecules Materials Heterogeneous Mixtures Homogeneous Mixtures Two or more visible phases: Granite, Sand, Chicken noodle soup, …. Solutions gas-gas: like air liquid, liquid: alcohol and water liquid, solid, salt water gas, liquid: selzer water

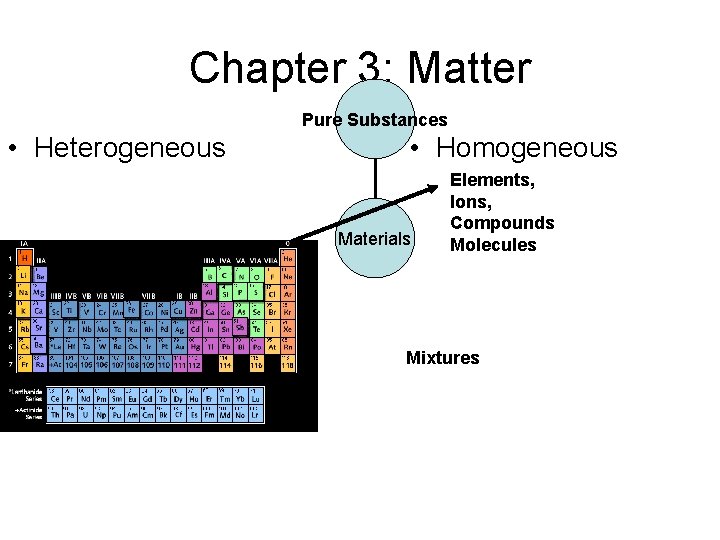
Chapter 3: Matter Pure Substances • Heterogeneous • Homogeneous Materials Elements, Ions, Compounds Molecules Heterogeneous Mixtures

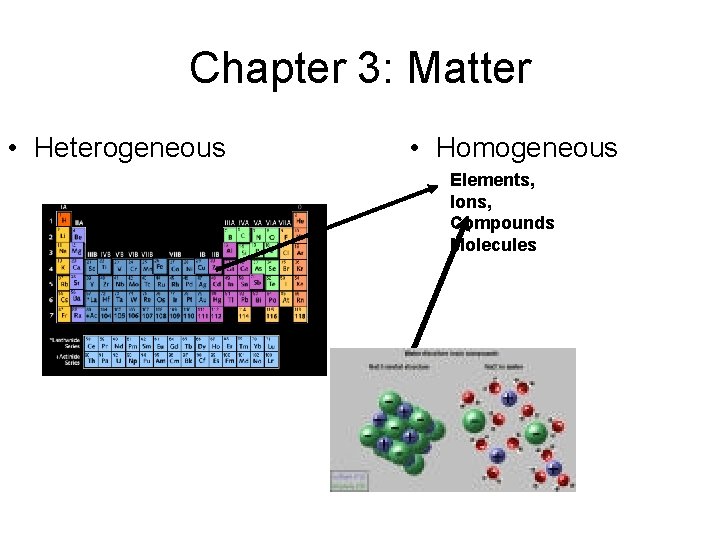
Chapter 3: Matter • Heterogeneous • Homogeneous Elements, Ions, Compounds Molecules Materials Heterogeneous Mixtures

Changes in Matter • Physical Change – – – Melting Boiling Freezing Condensing Crystallizing Dissolving • Chemical Change – Reacting with …. to make ….

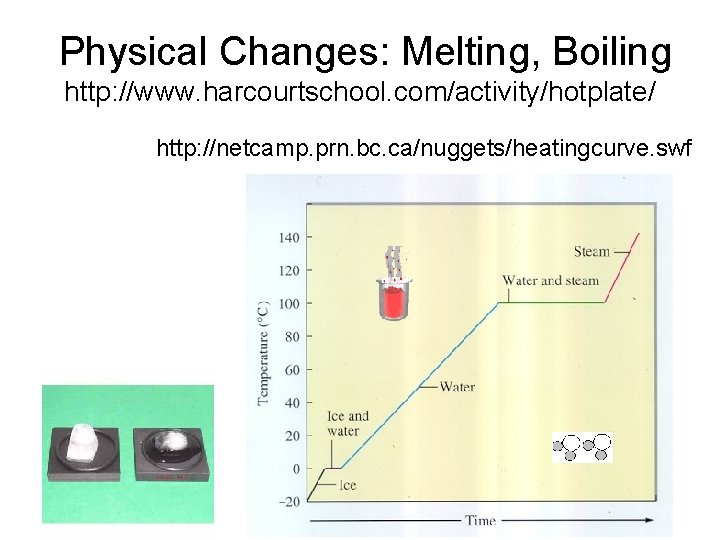
Physical Changes: Melting, Boiling http: //www. harcourtschool. com/activity/hotplate/ http: //netcamp. prn. bc. ca/nuggets/heatingcurve. swf

Physical Changes: Forming a crystal. http: //resourcescommittee. house. gov/subcommittees/emr/usgsweb/photogallery/ http: //stuff. ratjed. com/fluid%20 crystal%20 interaction%20 copy. jpg

Chemical Changes: These involve rearrangement of atoms within the compound/molecule!

Begin Preparing for Lab 5 • Each Group Member has a role: – – – Summarize Introduction --Write questions 1 - 4, 1, 2 Answer Pre-lab questions Make personalized procedure Copy data table HONORS: Do #1 going further. Procedure Initial observations Intermediate observations Final Physical properties. Melt birthday candle wax in test tube. (example) Green, opaque, waxy, cylindrical glows pale green, becomes the shape of the container Smaller volume, green, opaque, waxy. fills the bottom of container.