Rutherfords Gold Foil Experiment T T The Professional

- Slides: 7

Rutherford’s Gold Foil Experiment T. T. The Professional Development Service for Teachers is funded by the Department of Education and Skills under the National Development Plan

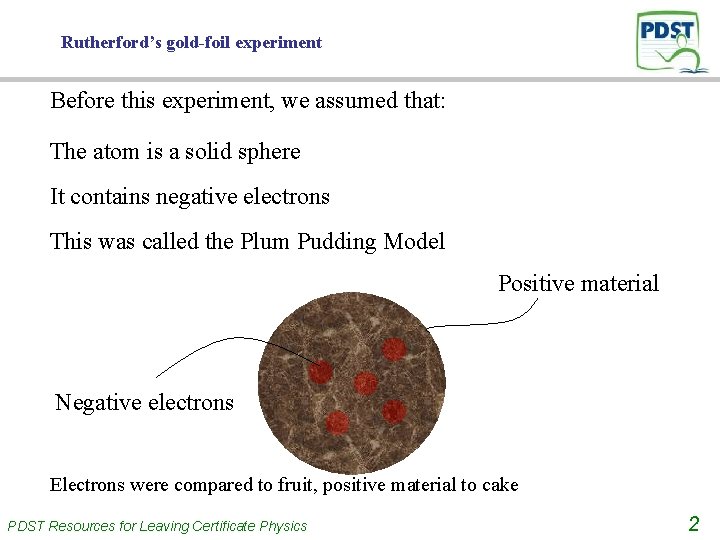
Rutherford’s gold-foil experiment Before this experiment, we assumed that: The atom is a solid sphere It contains negative electrons This was called the Plum Pudding Model Positive material Negative electrons Electrons were compared to fruit, positive material to cake PDST Resources for Leaving Certificate Physics 2

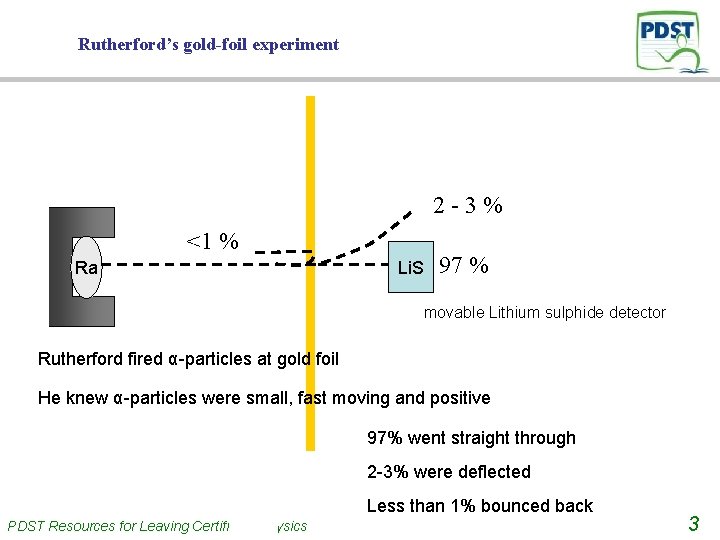
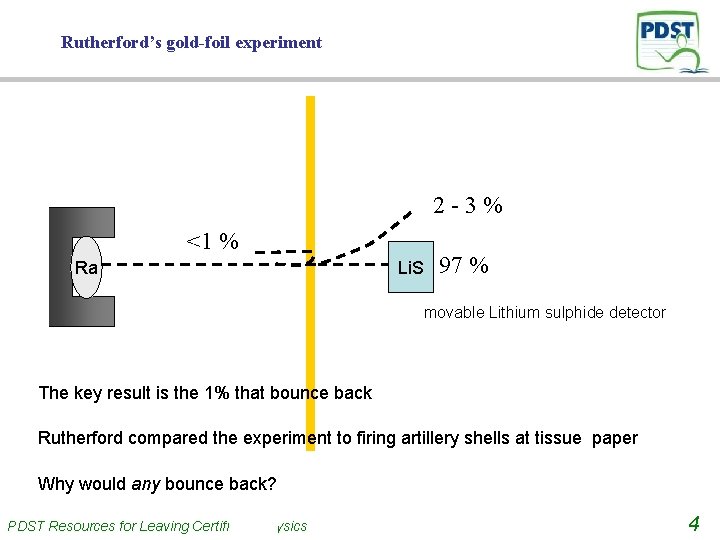
Rutherford’s gold-foil experiment 2 -3% <1 % Ra Li. S 97 % movable Lithium sulphide detector Rutherford fired α-particles at gold foil He knew α-particles were small, fast moving and positive 97% went straight through 2 -3% were deflected Less than 1% bounced back PDST Resources for Leaving Certificate Physics 3

Rutherford’s gold-foil experiment 2 -3% <1 % Ra Li. S 97 % movable Lithium sulphide detector The key result is the 1% that bounce back Rutherford compared the experiment to firing artillery shells at tissue paper Why would any bounce back? PDST Resources for Leaving Certificate Physics 4

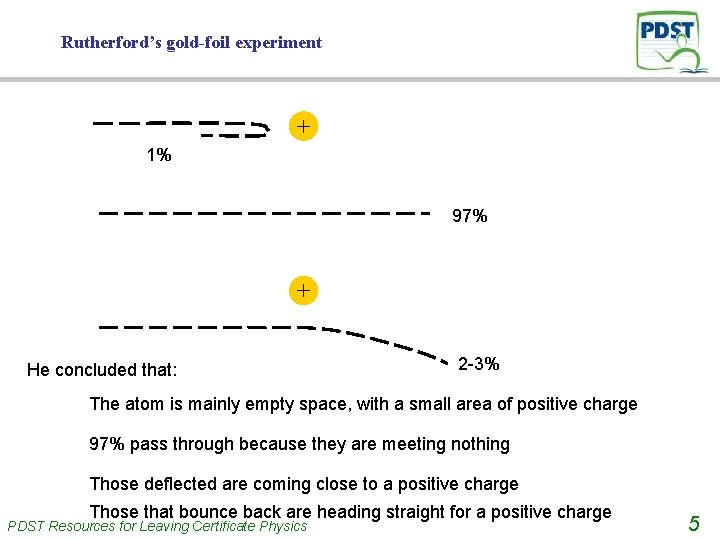
Rutherford’s gold-foil experiment + 1% 97% + He concluded that: 2 -3% The atom is mainly empty space, with a small area of positive charge 97% pass through because they are meeting nothing Those deflected are coming close to a positive charge Those that bounce back are heading straight for a positive charge PDST Resources for Leaving Certificate Physics 5

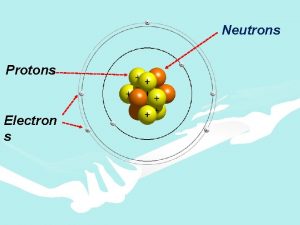
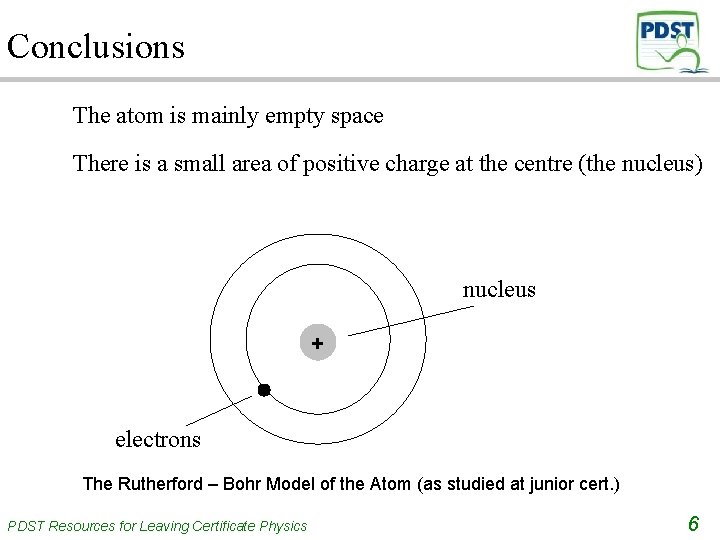
Conclusions The atom is mainly empty space There is a small area of positive charge at the centre (the nucleus) nucleus + electrons The Rutherford – Bohr Model of the Atom (as studied at junior cert. ) PDST Resources for Leaving Certificate Physics 6

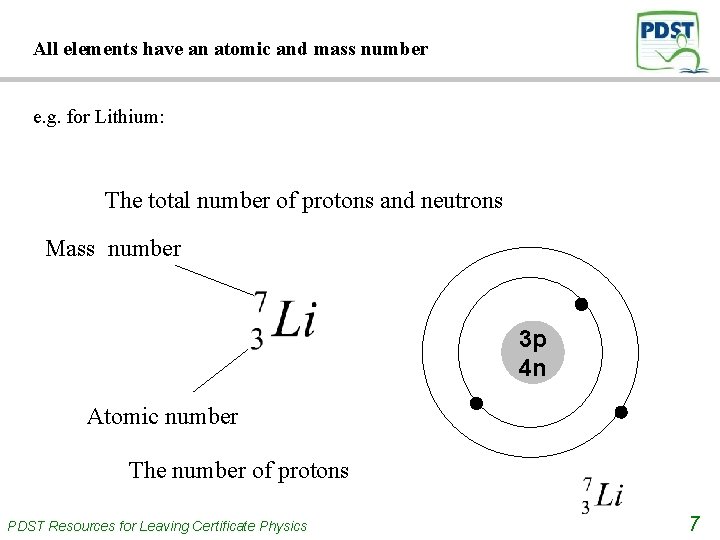
All elements have an atomic and mass number e. g. for Lithium: The total number of protons and neutrons Mass number 3 p 4 n Atomic number The number of protons PDST Resources for Leaving Certificate Physics 7