REMEDIATION OF WASTEWATER CONTAINING HEAVY METALS USING RAW








- Slides: 10

REMEDIATION OF WASTEWATER CONTAINING HEAVY METALS USING RAW AND MODIFIED DIATOMITE Miloš T. Nenadović

The main goal l To investigate structural changes in diatomite l Changes caused by mechanical milling with magnesium hydride l It is very important to know what is going on with lattice parametar of Mg-hydride.

There are two types of raw diatomite l The first one is CYLINDRICAL

The second one has rod like particles This two diatomites has the same chemical structure, but configuration is tottaly different. l Diatomite is completly natural product!!! l

What is happened next? Magnesium hydride was premilled for 10 hours l Then diatomite was added and the milling was performed for another 1 hour. l Milling speed of 850 rpm was chosen for the purpose of this study l And then we have got something like this!!!! l

Structural changes This is the sample Mg. H 2 -diatomite composite l Ratio Mg. H 2 : diatomite = 90 : 10 l

Structural changes This is also Mg. H 2 – diatomite composte but ratio between this two constituents is l Mg. H 2 : diatomite = 10 : 90 l

Structural changes l We can see some changes in the structure of diatomite and Mg-hydride l It is strongly depend of the ratio of constituents l The sample with 90 % of diatomite shows sponge like structure l It is better for sorption of heavy metals

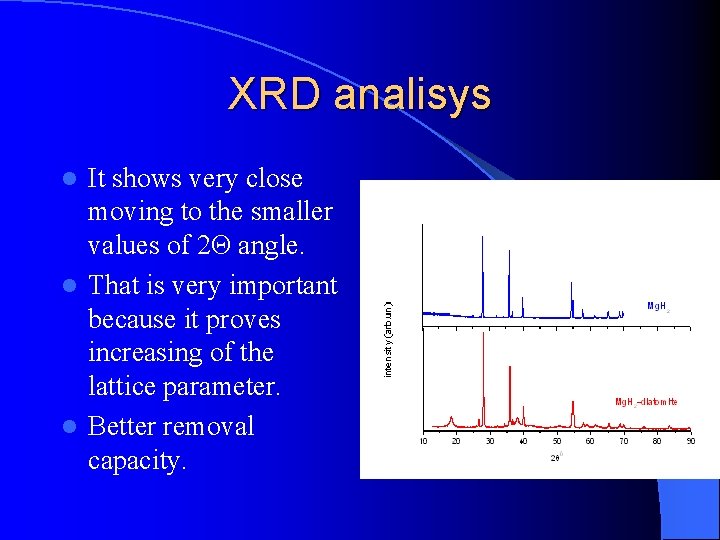
XRD analisys It shows very close moving to the smaller values of 2Θ angle. l That is very important because it proves increasing of the lattice parameter. l Better removal capacity. l

Conclusion l Investigation demonstrated that diatomite has a higher removal capacity for Pb 2+, Ni 2+, Cu 2+, Hg 2+ and Cd 2+ from water than untreated diatomite. The adsorption capacity of modified diatomite increased by decreasing the quantity of magnesium hydride deposited on the surface.
 Dropped like luxuries in a yelling alarm
Dropped like luxuries in a yelling alarm Ratey algebra 2
Ratey algebra 2 Ionic compounds containing transition metals
Ionic compounds containing transition metals Density of metalloids
Density of metalloids Properties of matter and materials grade 7
Properties of matter and materials grade 7 Periodic table with metals and nonmetals
Periodic table with metals and nonmetals Technology grade 7 term 3 notes
Technology grade 7 term 3 notes Ferrous metals
Ferrous metals Is sodium more reactive than potassium
Is sodium more reactive than potassium Remediation of the struggling medical learner
Remediation of the struggling medical learner Defect remediation
Defect remediation


















