Randomized Phase IIIII Clinical and Translational Trial of





- Slides: 11

Randomized Phase II/III Clinical and Translational Trial of Combination Checkpoint and IDO 1 Inhibition with Chemoradiotherapy/Radiotherapy for Newly Diagnosed Glioblastoma Rimas Lukas, MD Associate Chief, Neuro-Oncology Division Lou & Jean Malnati Brain Tumor Institute at the Lurie Comprehensive Cancer Center Northwestern University

IDO 1 is an IFNinducible enzyme that converts tryptophan into kynurenine

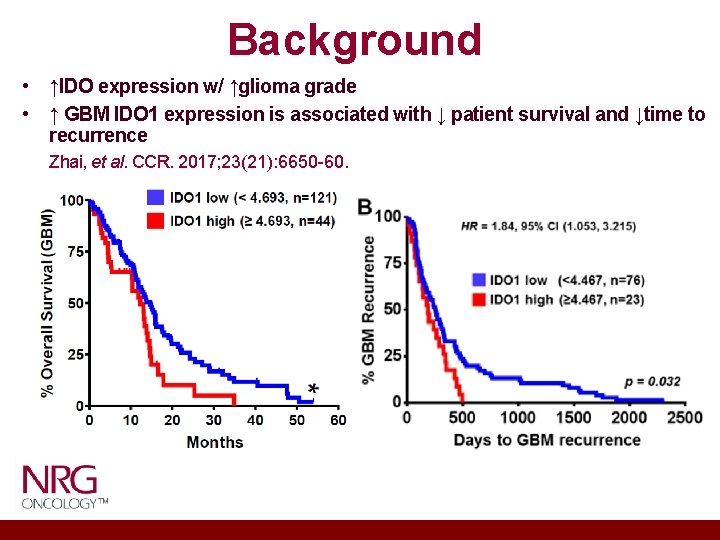
Background • ↑IDO expression w/ ↑glioma grade • ↑ GBM IDO 1 expression is associated with ↓ patient survival and ↓time to recurrence Zhai, et al. CCR. 2017; 23(21): 6650 -60.

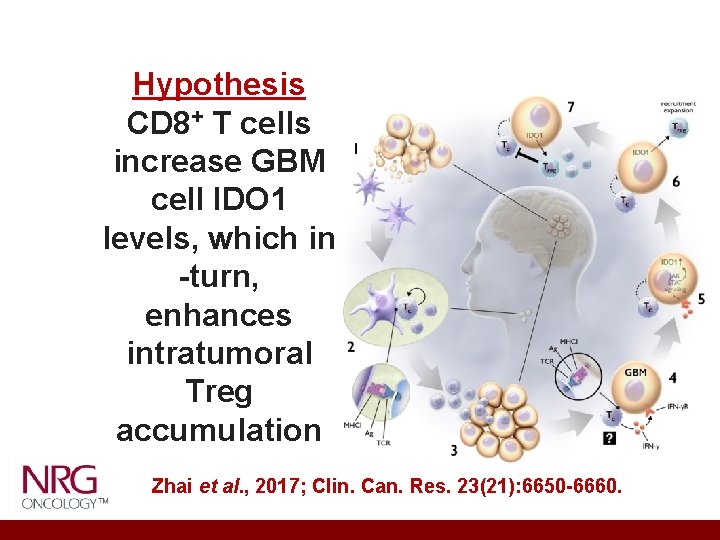
Hypothesis CD 8+ T cells increase GBM cell IDO 1 levels, which in -turn, enhances intratumoral Treg accumulation Zhai et al. , 2017; Clin. Can. Res. 23(21): 6650 -6660.

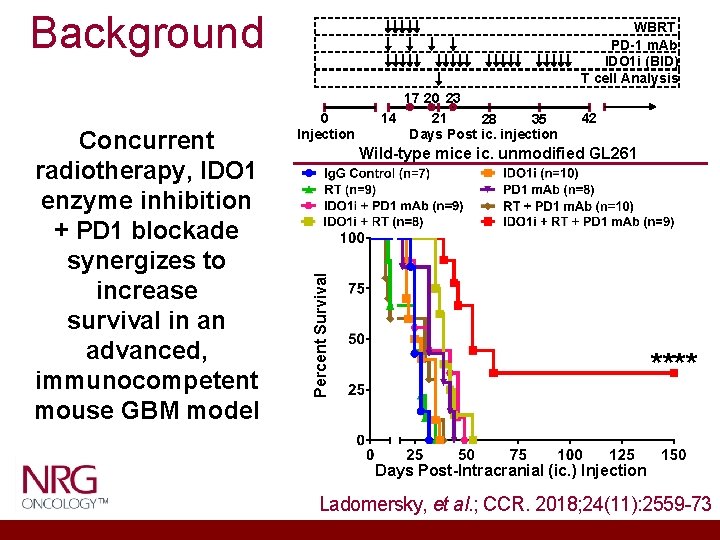
Background 0 Injection 17 20 23 21 14 35 28 Days Post ic. injection 42 Wild-type mice ic. unmodified GL 261 Percent Survival Concurrent radiotherapy, IDO 1 enzyme inhibition + PD 1 blockade synergizes to increase survival in an advanced, immunocompetent mouse GBM model WBRT PD-1 m. Ab IDO 1 i (BID) T cell Analysis **** Days Post-Intracranial (ic. ) Injection Ladomersky, et al. ; CCR. 2018; 24(11): 2559 -73


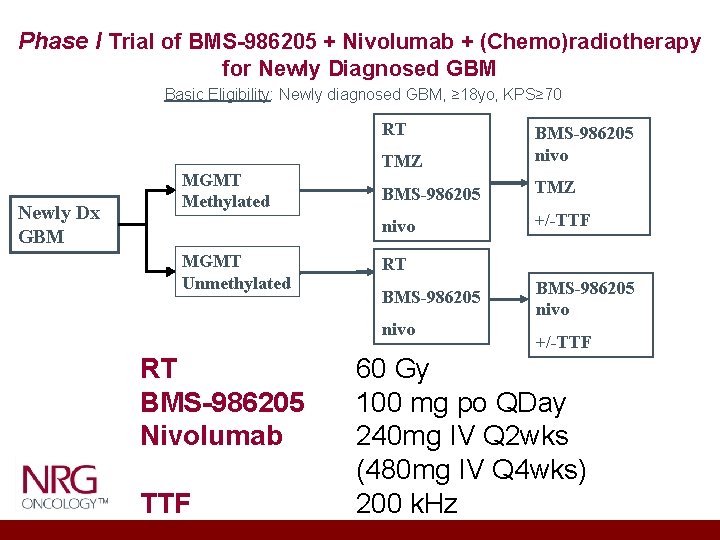
Phase I Trial of BMS-986205 + Nivolumab + (Chemo)radiotherapy for Newly Diagnosed GBM Basic Eligibility: Newly diagnosed GBM, ≥ 18 yo, KPS≥ 70 RT Newly Dx GBM MGMT Methylated MGMT Unmethylated TMZ BMS-986205 nivo BMS-986205 TMZ nivo +/-TTF RT BMS-986205 nivo RT BMS-986205 Nivolumab TTF BMS-986205 nivo +/-TTF 60 Gy 100 mg po QDay 240 mg IV Q 2 wks (480 mg IV Q 4 wks) 200 k. Hz

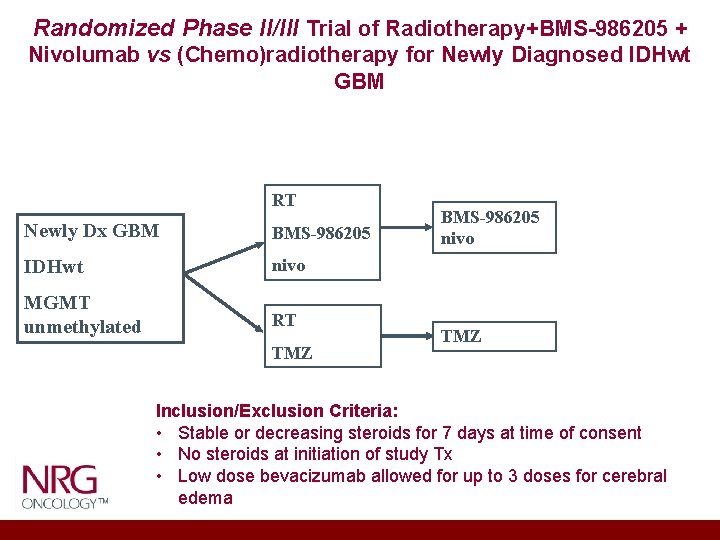
Randomized Phase II/III Trial of Radiotherapy+BMS-986205 + Nivolumab vs (Chemo)radiotherapy for Newly Diagnosed IDHwt GBM RT Newly Dx GBM BMS-986205 IDHwt nivo MGMT unmethylated RT TMZ BMS-986205 nivo TMZ Inclusion/Exclusion Criteria: • Stable or decreasing steroids for 7 days at time of consent • No steroids at initiation of study Tx • Low dose bevacizumab allowed for up to 3 doses for cerebral edema

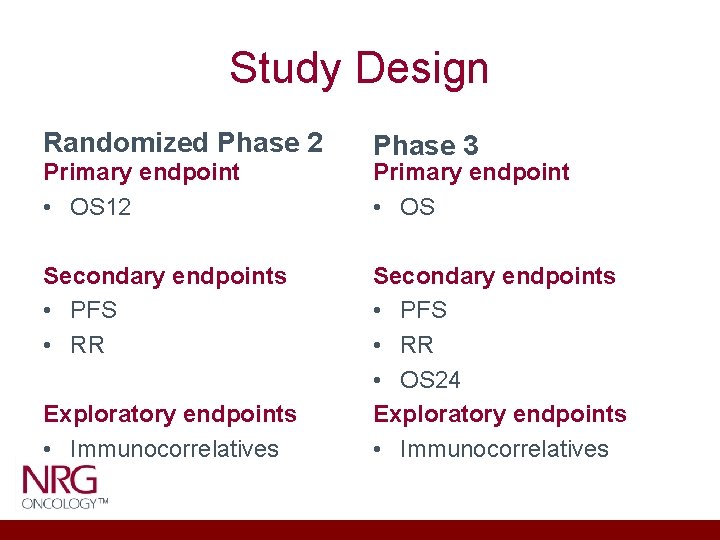
Study Design Randomized Phase 2 Primary endpoint • OS 12 Secondary endpoints • PFS • RR Exploratory endpoints • Immunocorrelatives Phase 3 Primary endpoint • OS Secondary endpoints • PFS • RR • OS 24 Exploratory endpoints • Immunocorrelatives

Points of Consideration • Incidence of pseudoprogression in newly Dx GBM Tx w/ immuno. Tx unreported • Use of non-radiographic endpoint allows for low-dose bev for cerebral edema (as opposed to steroids)

ACKNOWLEDGEMENTS NRG ONCOLOGY Minesh Mehta, MD & NRG Brain Committee Biostatistics Mei Polley, Ph. D WAYNE STATE Csaba Juhasz, MD Ph. D FUNDING SOURCES P 50 CA 221747 SPORE NORTHWESTERN UNIVERSITY Radiation Oncology Sean Sachdev, MD Vinai Gondi, MD Timothy Kruser, MD Neuro-Oncology Roger Stupp, MD Priya Kumthekar, MD Jeff Raizer, MD Karan Dixit, MD Sean Grimm, MD Neurosurgery Derek Wainwright, Ph. D Maciej Lesniak, MD C. David James, Ph. D Christina Amidei, Ph. D, APN Neuropathology Daniel Brat, MD Craig Horbinski, MD
 Advantage of randomized controlled trial
Advantage of randomized controlled trial Fsfd clinical trial
Fsfd clinical trial Rsna clinical trial processor
Rsna clinical trial processor Morpheus clinical trial
Morpheus clinical trial Clinical trial budget example
Clinical trial budget example Novel clinical drug trial design
Novel clinical drug trial design Clinical trials gov api
Clinical trials gov api Clinical trial financial management
Clinical trial financial management Phase 4 trial
Phase 4 trial Nida clinical trial network
Nida clinical trial network Companion diagnostic clinical trial
Companion diagnostic clinical trial Clinical trial exports
Clinical trial exports




















