Radical polymerization initiation propagation chain transfer termination start


![When the initiator decomposes slowly compared to the entire polymerization process: When ln([M]0/[M]) is When the initiator decomposes slowly compared to the entire polymerization process: When ln([M]0/[M]) is](https://slidetodoc.com/presentation_image_h2/cbcbe357562ba6d93284698ee60bd4e6/image-10.jpg)
![Kinetic chain length Also =kp [M] , with t time of growth of a Kinetic chain length Also =kp [M] , with t time of growth of a](https://slidetodoc.com/presentation_image_h2/cbcbe357562ba6d93284698ee60bd4e6/image-11.jpg)
- Slides: 11

Radical polymerization • • initiation propagation chain transfer termination (start) (growth) (stop/start) (stop) During initiation active centers are being formed. During termination active centers disappear. Concentration of active centers is very low (10 -9 - 10 -7 mol/L). Growth rate of a chain is very high (103 - 104 units/s). Chains with a degree of polymerization of 103 to 104 are being formed in 0. 1 to 10 s. 1

Initiation Most common method of initiation: thermal decomposition of a thermally labile compound, the initiator These radicals are called primary radicals. Initiation takes place by addition of a monomer unit. kd is a first order rate constant. Common values of kd are in the range: 10 -4 - 10 -6 s-1. The rate of radical production is then given by: 2

The actual rate of initiation Ri is expressed in terms of the rate of radical production that leads to actual polymer chains growing!: where f is the efficiency factor: the fraction of radicals that really leads to initiation. Therefore, in all cases: f 1 The rate constant ki is not used in the mathematical description of the polymerization. Examples of thermal initiators: Ea = 140 – 160 k. J mol-1 3

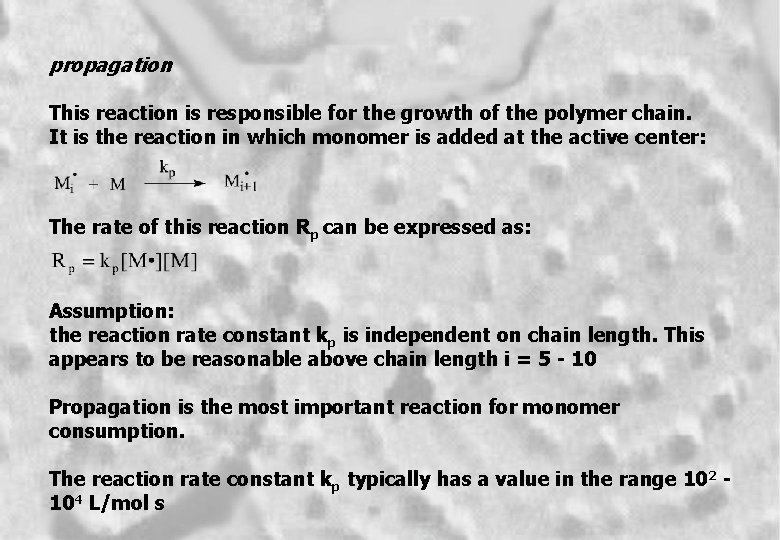
propagation This reaction is responsible for the growth of the polymer chain. It is the reaction in which monomer is added at the active center: The rate of this reaction Rp can be expressed as: Assumption: the reaction rate constant kp is independent on chain length. This appears to be reasonable above chain length i = 5 - 10 Propagation is the most important reaction for monomer consumption. The reaction rate constant kp typically has a value in the range 102 104 L/mol s 4

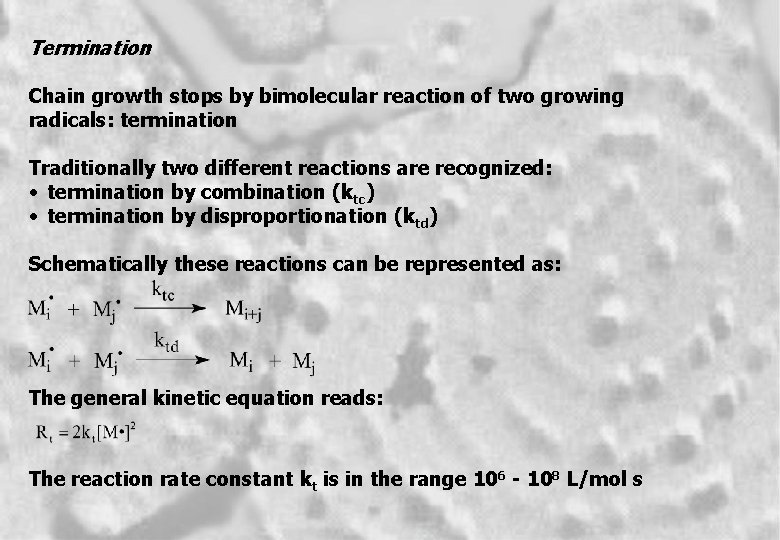
Termination Chain growth stops by bimolecular reaction of two growing radicals: termination Traditionally two different reactions are recognized: • termination by combination (ktc) • termination by disproportionation (ktd) Schematically these reactions can be represented as: The general kinetic equation reads: The reaction rate constant kt is in the range 106 - 108 L/mol s 5

Termination Every reaction consists of two steps: 1) approach of both reactants 2) chemical reaction The second step in the termination reaction is very fast. This means that the rate of approach (partially) determines the overall termination rate. 6

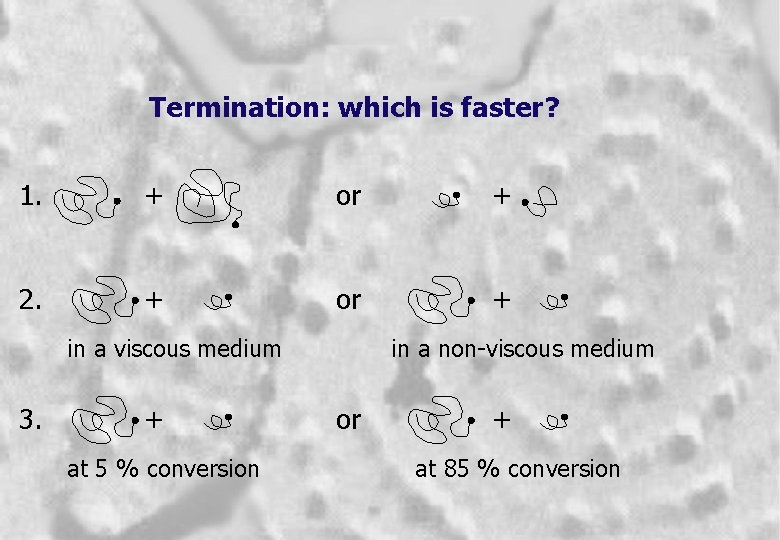
Termination: which is faster? 1. + or + 2. + or + in a viscous medium 3. + at 5 % conversion in a non-viscous medium or + at 85 % conversion 7

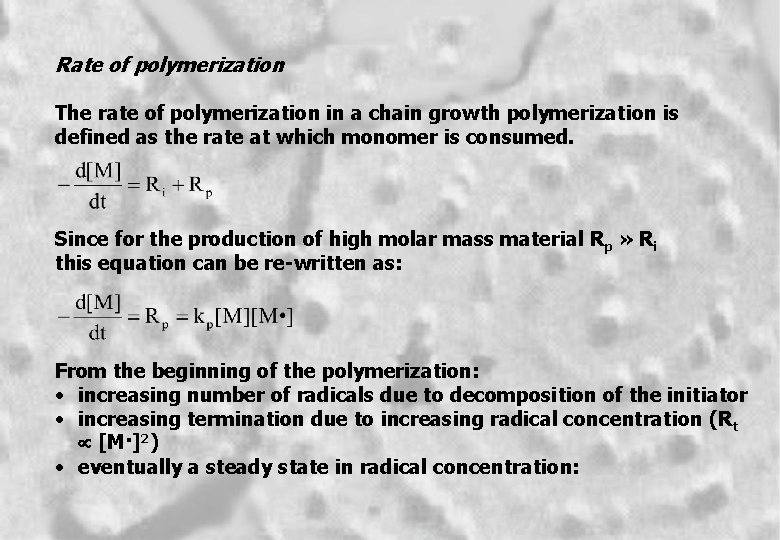
Rate of polymerization The rate of polymerization in a chain growth polymerization is defined as the rate at which monomer is consumed. Since for the production of high molar mass material Rp » Ri this equation can be re-written as: From the beginning of the polymerization: • increasing number of radicals due to decomposition of the initiator • increasing termination due to increasing radical concentration (R t µ [M·]2) • eventually a steady state in radical concentration: 8

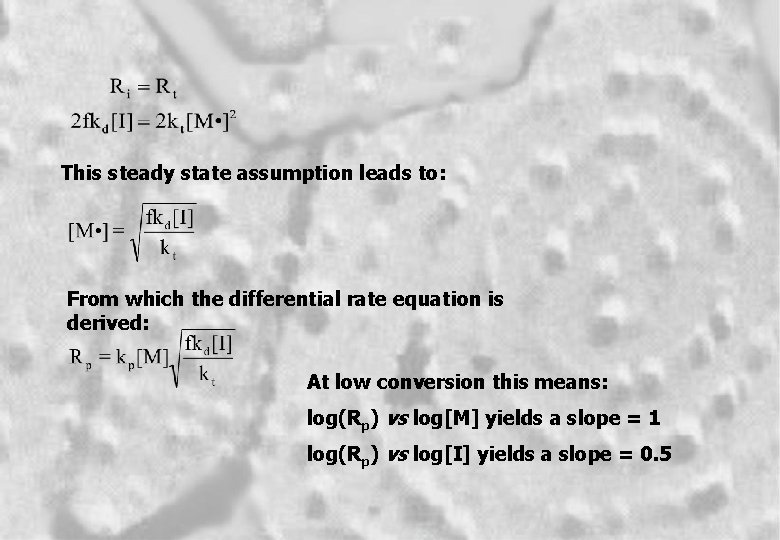
This steady state assumption leads to: From which the differential rate equation is derived: At low conversion this means: log(Rp) vs log[M] yields a slope = 1 log(Rp) vs log[I] yields a slope = 0. 5 9
![When the initiator decomposes slowly compared to the entire polymerization process When lnM0M is When the initiator decomposes slowly compared to the entire polymerization process: When ln([M]0/[M]) is](https://slidetodoc.com/presentation_image_h2/cbcbe357562ba6d93284698ee60bd4e6/image-10.jpg)
When the initiator decomposes slowly compared to the entire polymerization process: When ln([M]0/[M]) is plotted versus time, then the slope should equal k: 10
![Kinetic chain length Also kp M with t time of growth of a Kinetic chain length Also =kp [M] , with t time of growth of a](https://slidetodoc.com/presentation_image_h2/cbcbe357562ba6d93284698ee60bd4e6/image-11.jpg)
Kinetic chain length Also =kp [M] , with t time of growth of a polymer chain Here we find at low conversion: log(n) vs log[M] yields a straight line; slope = 1 log (n) vs log[I] similar; slope = -0. 5 thus: increase in [I] leads to an increase in rate of polymerization and a decrease in chain length. The special case with a slowly decomposing initiator leads here to: where 11