Quiz 21405 Helpful Hint Name all of the


- Slides: 13

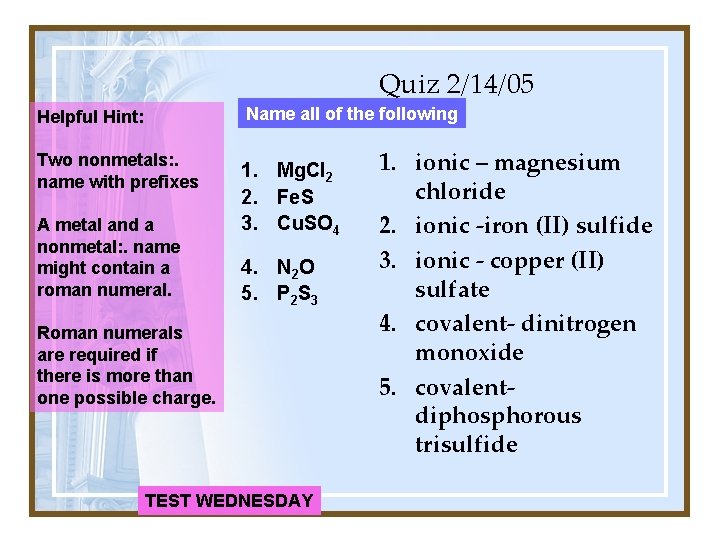
Quiz 2/14/05 Helpful Hint: Name all of the following Two nonmetals: . name with prefixes 1. Mg. Cl 2 2. Fe. S 3. Cu. SO 4 A metal and a nonmetal: . name might contain a roman numeral. 4. N 2 O 5. P 2 S 3 Roman numerals are required if there is more than one possible charge. TEST WEDNESDAY 1. ionic – magnesium chloride 2. ionic -iron (II) sulfide 3. ionic - copper (II) sulfate 4. covalent- dinitrogen monoxide 5. covalentdiphosphorous trisulfide

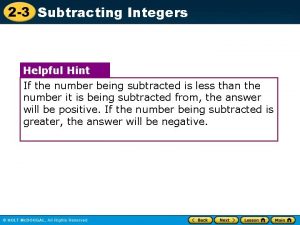
Lab 21: Due Thursday Feb 17 Remember: Ionic substances conduct electricity. Covalent substances do not. TEST Chapter 7: Wednesday Feb 16

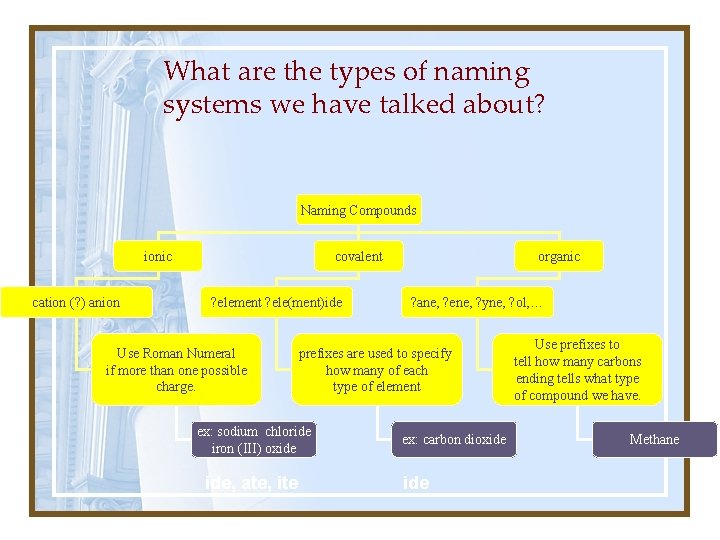
What are the types of naming systems we have talked about? Naming Compounds ionic cation (? ) anion covalent ? element ? ele(ment)ide Use Roman Numeral if more than one possible charge. ? ane, ? ene, ? yne, ? ol, … prefixes are used to specify how many of each type of element ex: sodium chloride iron (III) oxide ide, ate, ite organic ex: carbon dioxide Use prefixes to tell how many carbons ending tells what type of compound we have. Methane

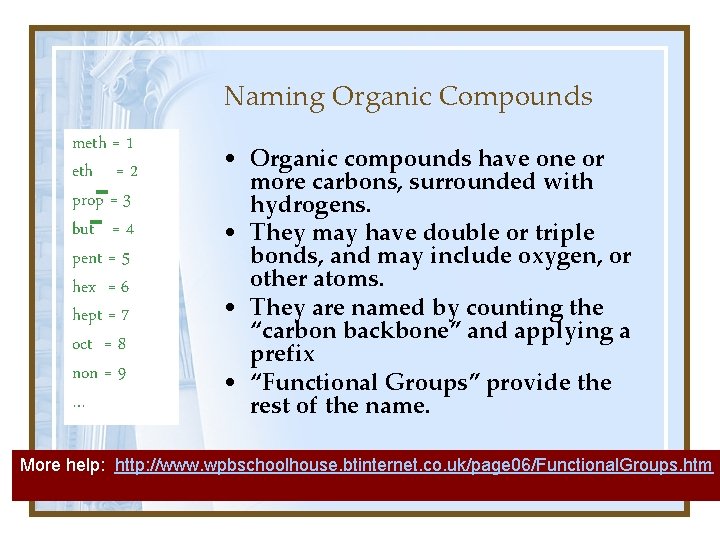
Naming Organic Compounds meth = 1 eth = 2 prop = 3 but = 4 pent = 5 hex = 6 hept = 7 oct = 8 non = 9 … • Organic compounds have one or more carbons, surrounded with hydrogens. • They may have double or triple bonds, and may include oxygen, or other atoms. • They are named by counting the “carbon backbone” and applying a prefix • “Functional Groups” provide the rest of the name. More help: http: //www. wpbschoolhouse. btinternet. co. uk/page 06/Functional. Groups. htm

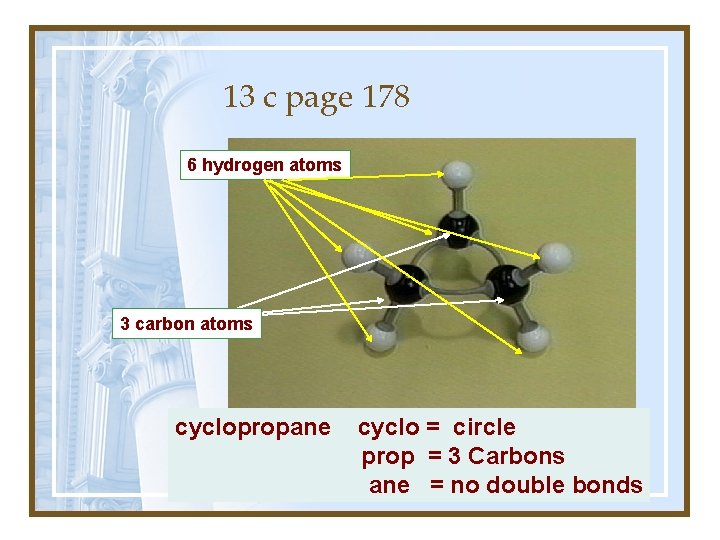
13 c page 178 6 hydrogen atoms 3 carbon atoms cyclopropane cyclo = circle prop = 3 Carbons What would this be called? ane = no double bonds

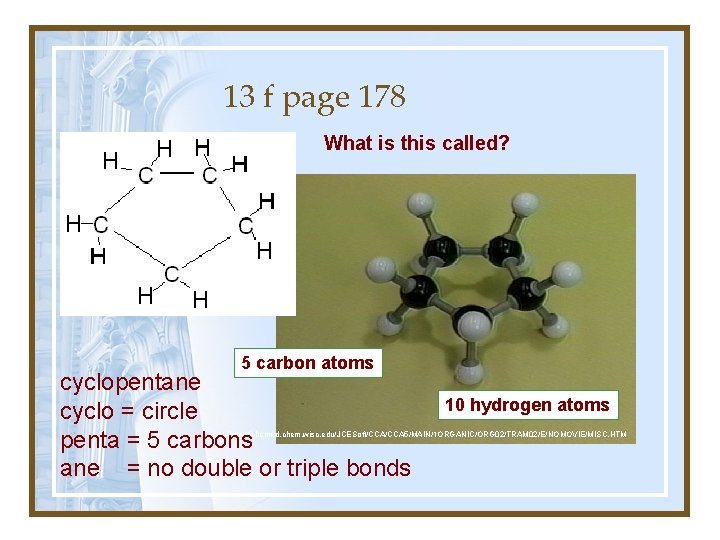
13 f page 178 What is this called? 5 carbon atoms cyclopentane cyclo = circle penta = 5 carbons ane = no double or triple bonds 10 hydrogen atoms http: //jchemed. chem. wisc. edu/JCESoft/CCA 5/MAIN/1 ORGANIC/ORG 02/TRAM 02/E/NOMOVIE/MISC. HTM

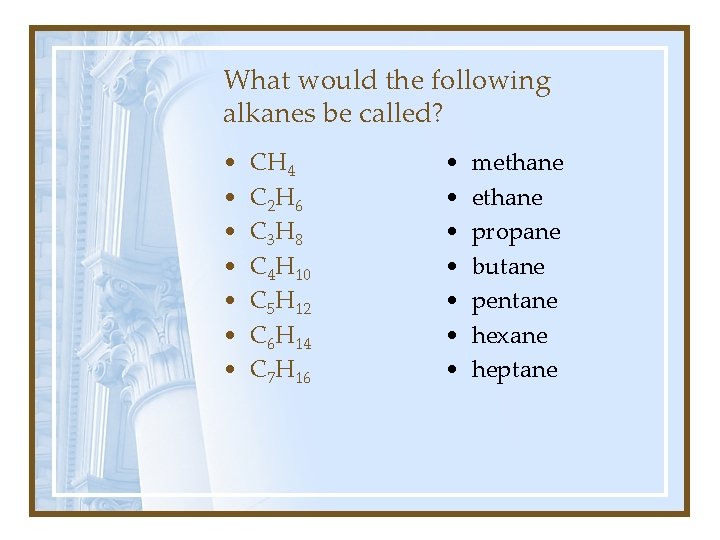
What would the following alkanes be called? • • CH 4 C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12 C 6 H 14 C 7 H 16 • • methane propane butane pentane hexane heptane

What would the following alkenes be called? • • • C 2 H 4 C 3 H 6 C 4 H 8 C 5 H 10 C 6 H 12 C 7 H 14 • • • ethene propene butene pentene hexene heptene Notice that there is no CH 2. Why can’t there be? There is nothing for the carbon to double bond with!

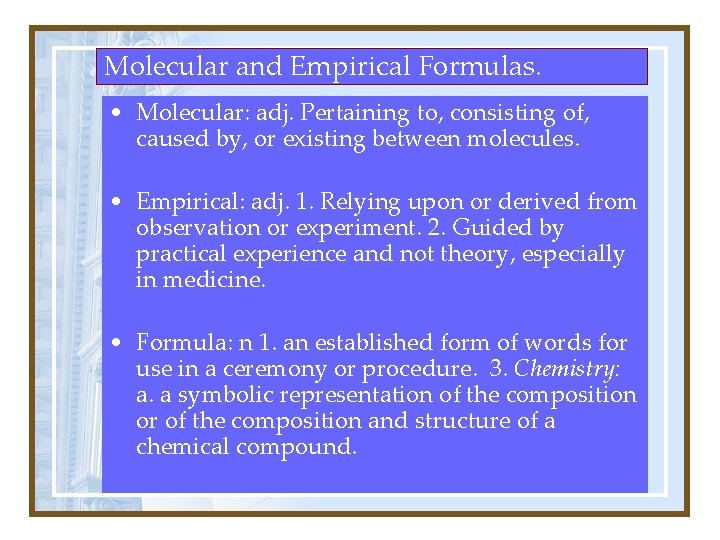
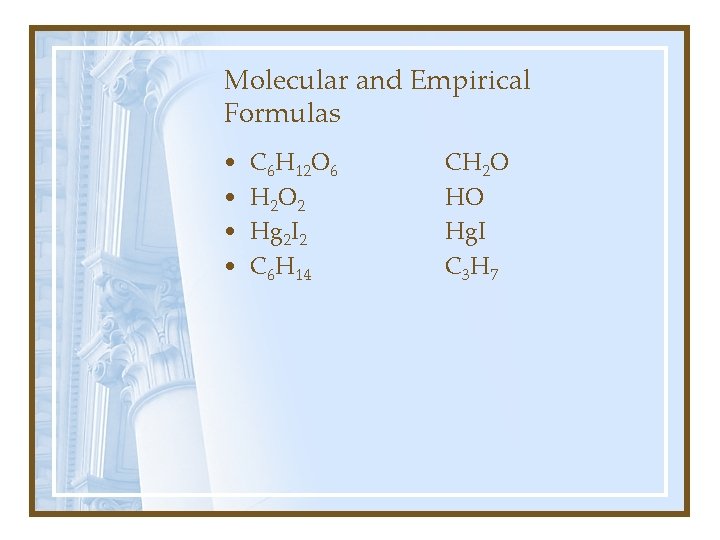
Molecular and Empirical Formulas. • Molecular: adj. Pertaining to, consisting of, caused by, or existing between molecules. • Empirical: adj. 1. Relying upon or derived from observation or experiment. 2. Guided by practical experience and not theory, especially in medicine. • Formula: n 1. an established form of words for use in a ceremony or procedure. 3. Chemistry: a. a symbolic representation of the composition or of the composition and structure of a chemical compound.

Molecular and Empirical Formulas • • C 6 H 12 O 6 H 2 O 2 Hg 2 I 2 C 6 H 14 CH 2 O HO Hg. I C 3 H 7

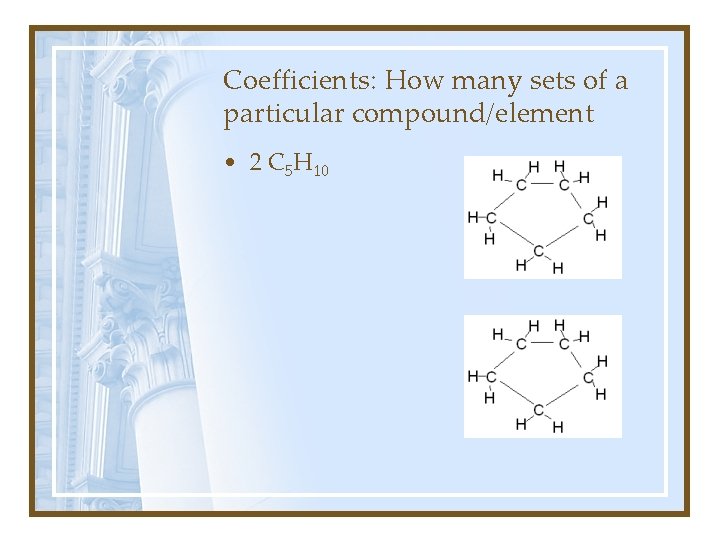
Coefficients: How many sets of a particular compound/element • 2 C 5 H 10

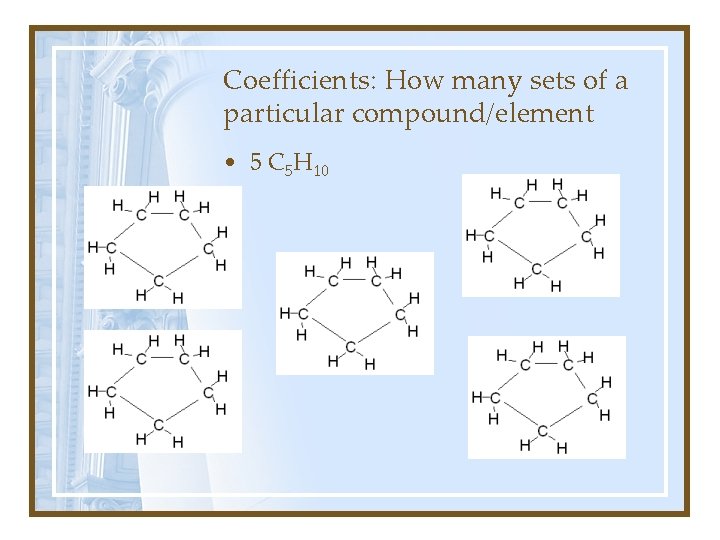
Coefficients: How many sets of a particular compound/element • 5 C 5 H 10

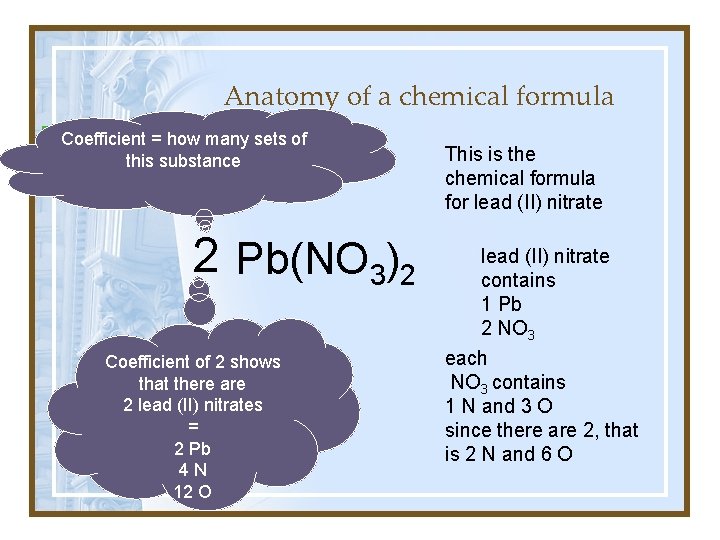
Anatomy of a chemical formula Coefficient = how of many of What is the name thissets compound? thisofsubstance How many each atom are there? 2 Pb(NO 3)2 Coefficient of 2 shows that there are 2 lead (II) nitrates = 2 Pb 4 N 12 O This is the chemical formula for lead (II) nitrate contains 1 Pb 2 NO 3 each NO 3 contains 1 N and 3 O since there are 2, that is 2 N and 6 O
 Name
Name Helpful hint
Helpful hint A good or helpful result or effect in research
A good or helpful result or effect in research Are microorganisms helpful or harmful
Are microorganisms helpful or harmful A scout is loyal
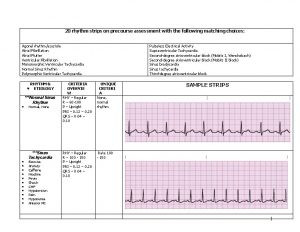
A scout is loyal Rhythm identification
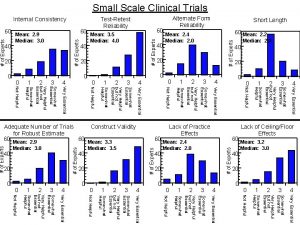
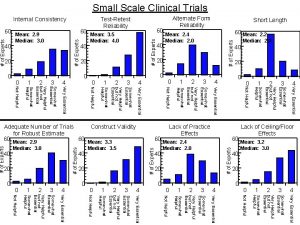
Rhythm identification Helpful values
Helpful values Gsp teacher recommendation
Gsp teacher recommendation Hope you find this helpful
Hope you find this helpful Pre-writing definition
Pre-writing definition Intermittent schedule of reinforcement
Intermittent schedule of reinforcement Positive, constructive, helpful behavior
Positive, constructive, helpful behavior Variable interval schedule
Variable interval schedule Not very helpful
Not very helpful