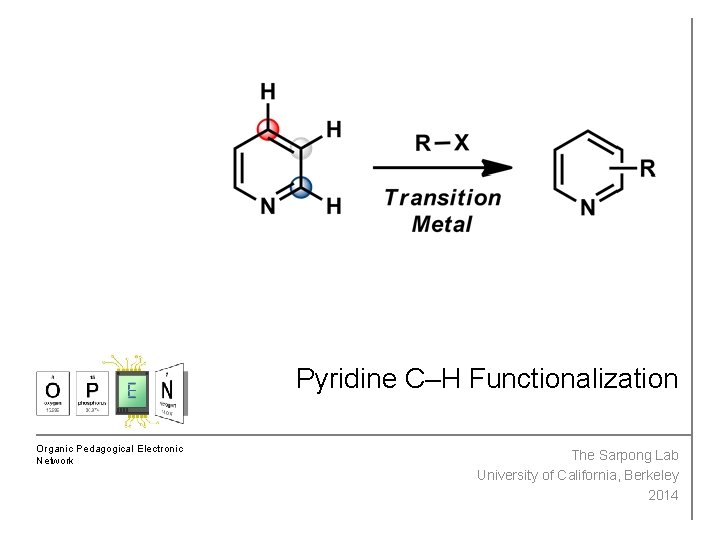
Pyridine CH Functionalization Organic Pedagogical Electronic Network The

- Slides: 13

Pyridine C–H Functionalization Organic Pedagogical Electronic Network The Sarpong Lab University of California, Berkeley 2014

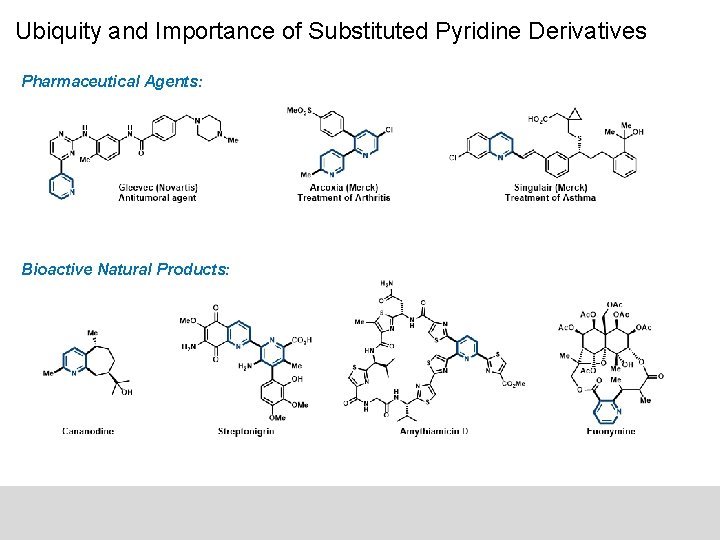
Ubiquity and Importance of Substituted Pyridine Derivatives Pharmaceutical Agents: Bioactive Natural Products:

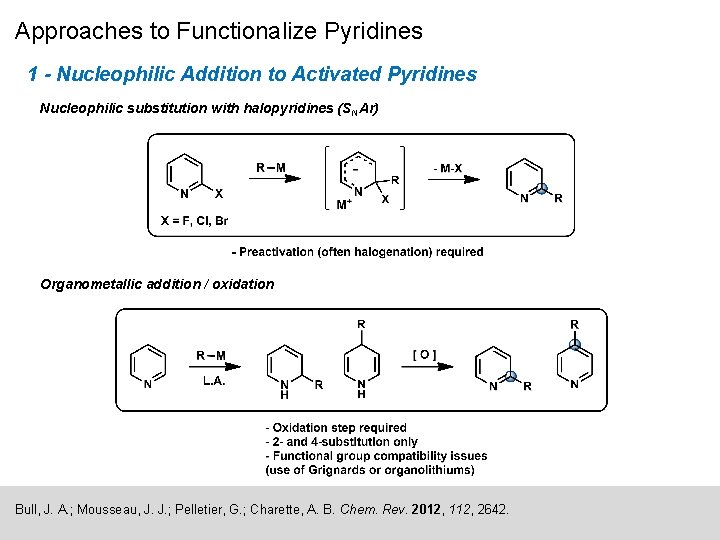
Approaches to Functionalize Pyridines 1 - Nucleophilic Addition to Activated Pyridines Nucleophilic substitution with halopyridines (SNAr) Organometallic addition / oxidation Bull, J. A. ; Mousseau, J. J. ; Pelletier, G. ; Charette, A. B. Chem. Rev. 2012, 112, 2642.

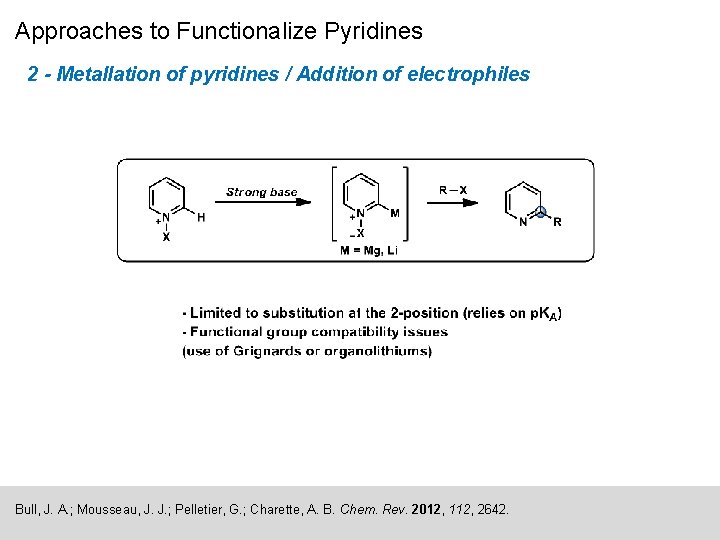
Approaches to Functionalize Pyridines 2 - Metallation of pyridines / Addition of electrophiles Bull, J. A. ; Mousseau, J. J. ; Pelletier, G. ; Charette, A. B. Chem. Rev. 2012, 112, 2642.

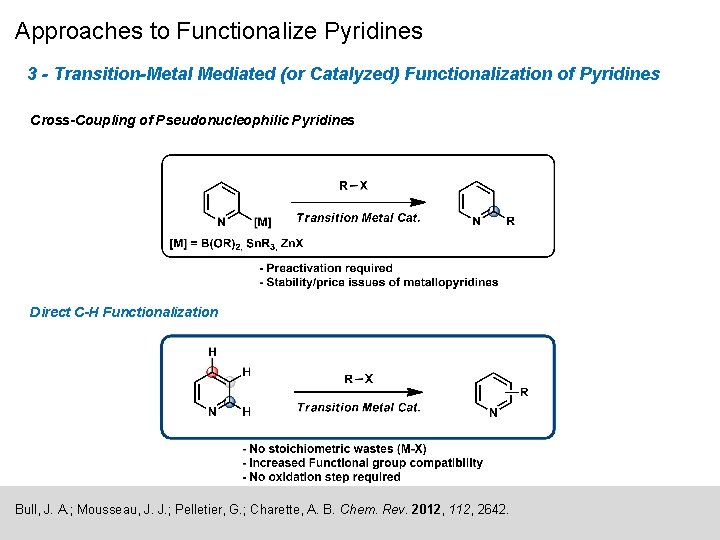
Approaches to Functionalize Pyridines 3 - Transition-Metal Mediated (or Catalyzed) Functionalization of Pyridines Cross-Coupling of Pseudonucleophilic Pyridines Direct C-H Functionalization Bull, J. A. ; Mousseau, J. J. ; Pelletier, G. ; Charette, A. B. Chem. Rev. 2012, 112, 2642.

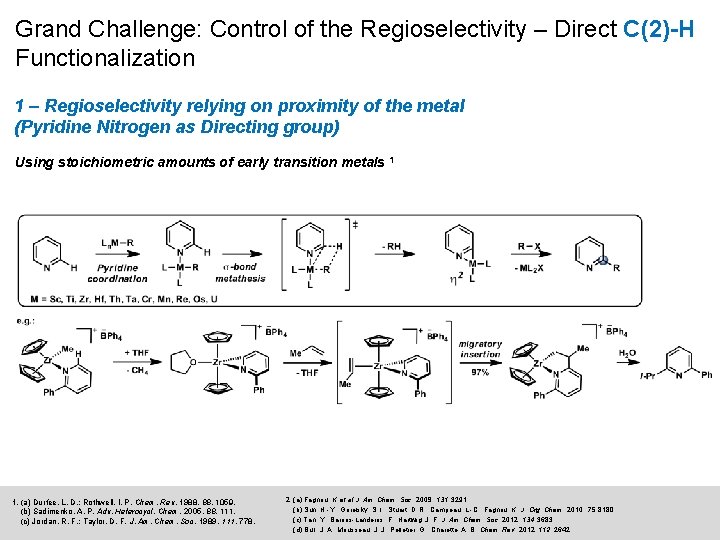
Grand Challenge: Control of the Regioselectivity – Direct C(2)-H Functionalization 1 – Regioselectivity relying on proximity of the metal (Pyridine Nitrogen as Directing group) Using stoichiometric amounts of early transition metals 1 1. (a) Durfee, L. D. ; Rothwell, I. P. Chem. Rev. 1988, 1059. (b) Sadimenko, A. P. Adv. Heterocycl. Chem. 2005, 88, 111. (c) Jordan, R. F. ; Taylor, D. F. J. Am. Chem. Soc. 1989, 111, 778. 2. (a) Fagnou, K. et al J. Am. Chem. Soc. 2009, 131, 3291. (b) Sun, H. -Y. ; Gorelsky, S. I. ; Stuart, D. R. ; Campeau, L. -C. ; Fagnou, K. J. Org. Chem. 2010, 75, 8180. (c) Tan, Y. ; Barrios-Landeros, F. ; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 3683. (d) Bull, J. A. ; Mousseau, J. J. ; Pelletier, G. ; Charette, A. B. Chem. Rev. 2012, 112, 2642.

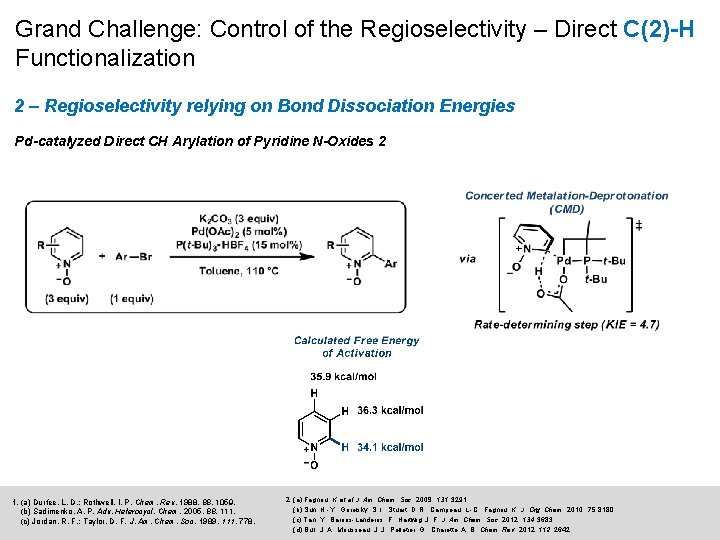
Grand Challenge: Control of the Regioselectivity – Direct C(2)-H Functionalization 2 – Regioselectivity relying on Bond Dissociation Energies Pd-catalyzed Direct CH Arylation of Pyridine N-Oxides 2 1. (a) Durfee, L. D. ; Rothwell, I. P. Chem. Rev. 1988, 1059. (b) Sadimenko, A. P. Adv. Heterocycl. Chem. 2005, 88, 111. (c) Jordan, R. F. ; Taylor, D. F. J. Am. Chem. Soc. 1989, 111, 778. 2. (a) Fagnou, K. et al J. Am. Chem. Soc. 2009, 131, 3291. (b) Sun, H. -Y. ; Gorelsky, S. I. ; Stuart, D. R. ; Campeau, L. -C. ; Fagnou, K. J. Org. Chem. 2010, 75, 8180. (c) Tan, Y. ; Barrios-Landeros, F. ; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 3683. (d) Bull, J. A. ; Mousseau, J. J. ; Pelletier, G. ; Charette, A. B. Chem. Rev. 2012, 112, 2642.

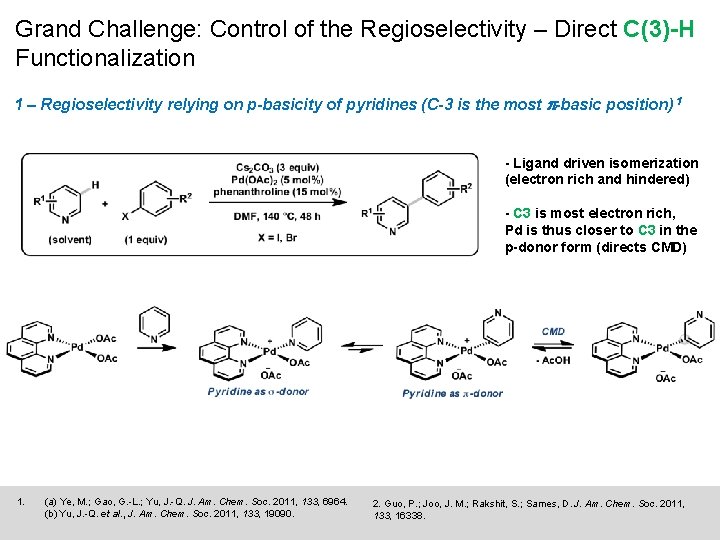
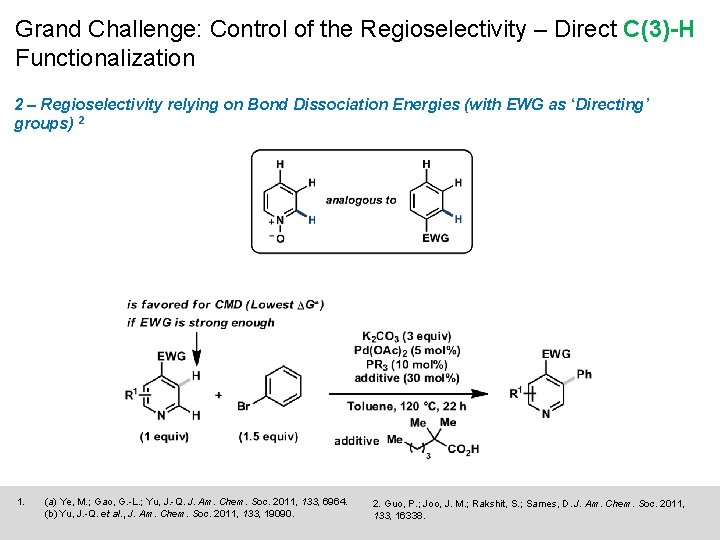
Grand Challenge: Control of the Regioselectivity – Direct C(3)-H Functionalization 1 – Regioselectivity relying on p-basicity of pyridines (C-3 is the most p-basic position) 1 - Ligand driven isomerization (electron rich and hindered) - C 3 is most electron rich, Pd is thus closer to C 3 in the p-donor form (directs CMD) 1. (a) Ye, M. ; Gao, G. -L. ; Yu, J. -Q. J. Am. Chem. Soc. 2011, 133, 6964. (b) Yu, J. -Q. et al. , J. Am. Chem. Soc. 2011, 133, 19090. 2. Guo, P. ; Joo, J. M. ; Rakshit, S. ; Sames, D. J. Am. Chem. Soc. 2011, 133, 16338.

Grand Challenge: Control of the Regioselectivity – Direct C(3)-H Functionalization 2 – Regioselectivity relying on Bond Dissociation Energies (with EWG as ‘Directing’ groups) 2 1. (a) Ye, M. ; Gao, G. -L. ; Yu, J. -Q. J. Am. Chem. Soc. 2011, 133, 6964. (b) Yu, J. -Q. et al. , J. Am. Chem. Soc. 2011, 133, 19090. 2. Guo, P. ; Joo, J. M. ; Rakshit, S. ; Sames, D. J. Am. Chem. Soc. 2011, 133, 16338.

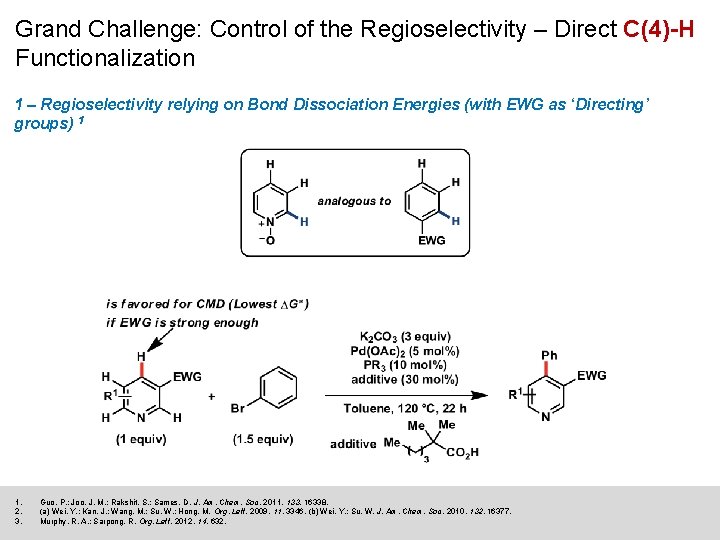
Grand Challenge: Control of the Regioselectivity – Direct C(4)-H Functionalization 1 – Regioselectivity relying on Bond Dissociation Energies (with EWG as ‘Directing’ groups) 1 1. 2. 3. Guo, P. ; Joo, J. M. ; Rakshit, S. ; Sames, D. J. Am. Chem. Soc. 2011, 133, 16338. (a) Wei, Y. ; Kan, J. ; Wang, M. ; Su, W. ; Hong, M. Org. Lett. 2009, 11, 3346. (b) Wei, Y. ; Su, W. J. Am. Chem. Soc. 2010, 132, 16377. Murphy, R. A. ; Sarpong, R. Org. Lett. 2012, 14, 632.

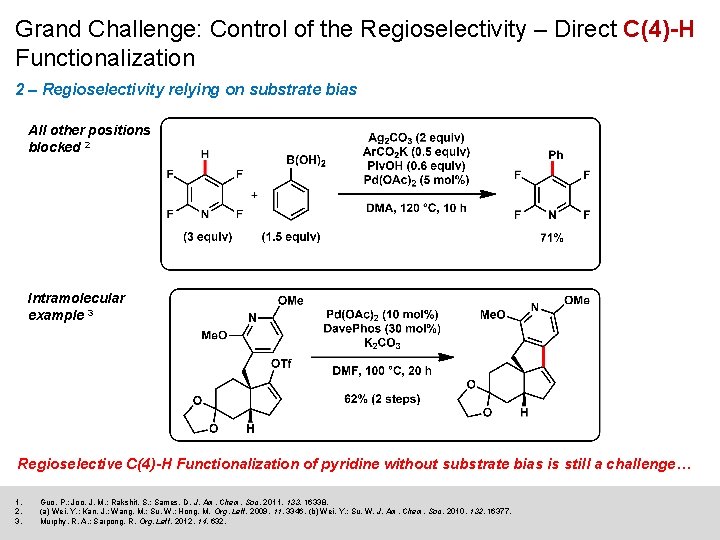
Grand Challenge: Control of the Regioselectivity – Direct C(4)-H Functionalization 2 – Regioselectivity relying on substrate bias All other positions blocked 2 Intramolecular example 3 Regioselective C(4)-H Functionalization of pyridine without substrate bias is still a challenge… 1. 2. 3. Guo, P. ; Joo, J. M. ; Rakshit, S. ; Sames, D. J. Am. Chem. Soc. 2011, 133, 16338. (a) Wei, Y. ; Kan, J. ; Wang, M. ; Su, W. ; Hong, M. Org. Lett. 2009, 11, 3346. (b) Wei, Y. ; Su, W. J. Am. Chem. Soc. 2010, 132, 16377. Murphy, R. A. ; Sarpong, R. Org. Lett. 2012, 14, 632.

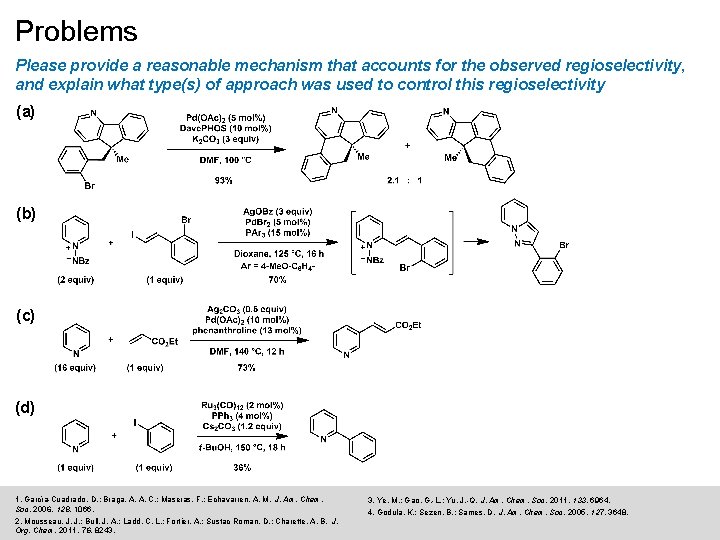
Problems Please provide a reasonable mechanism that accounts for the observed regioselectivity, and explain what type(s) of approach was used to control this regioselectivity (a) (b) (c) (d) 1. García-Cuadrado, D. ; Braga, A. A. C. ; Maseras, F. ; Echavarren, A. M. J. Am. Chem. Soc. 2006, 128, 1066. 2. Mousseau, J. J. ; Bull, J. A. ; Ladd, C. L. ; Fortier, A. ; Sustac Roman, D. ; Charette, A. B. J. Org. Chem. 2011, 76, 8243. 3. Ye, M. ; Gao, G. -L. ; Yu, J. -Q. J. Am. Chem. Soc. 2011, 133, 6964. 4. Godula, K. ; Sezen, B. ; Sames, D. J. Am. Chem. Soc. 2005, 127, 3648.

Contributed by: The Sarpong Lab University of California, Berkeley, 2014 This work is licensed under a Creative Commons Attribution. Share. Alike 4. 0 International License.