Properties of Fluids Fluids A fluid is anything









- Slides: 19

Properties of Fluids

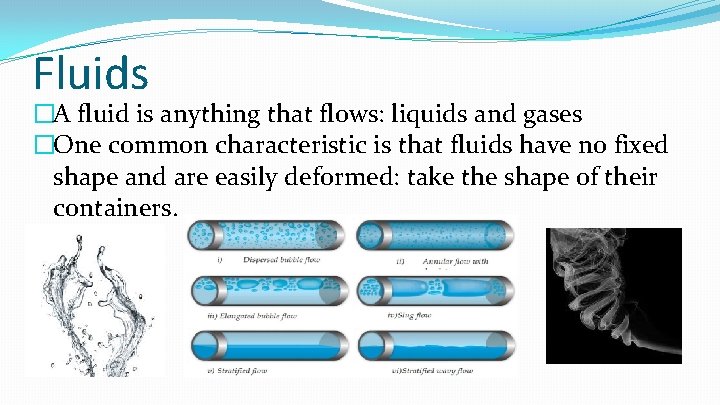
Fluids �A fluid is anything that flows: liquids and gases �One common characteristic is that fluids have no fixed shape and are easily deformed: take the shape of their containers.

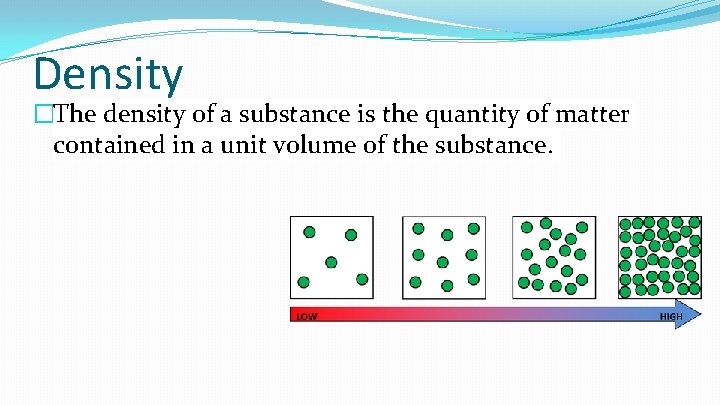
Density �The density of a substance is the quantity of matter contained in a unit volume of the substance.

Temperature �It is the property that determines the degree of hotness or coldness or the level of heat intensity of a fluid. Temperature is measured by using temperature scales. There are 3 commonly used temperature scales. They are 1. Celsius (or centigrade) scale 2. Fahrenheit scale 3. Kelvin scale (or absolute temperature scale)

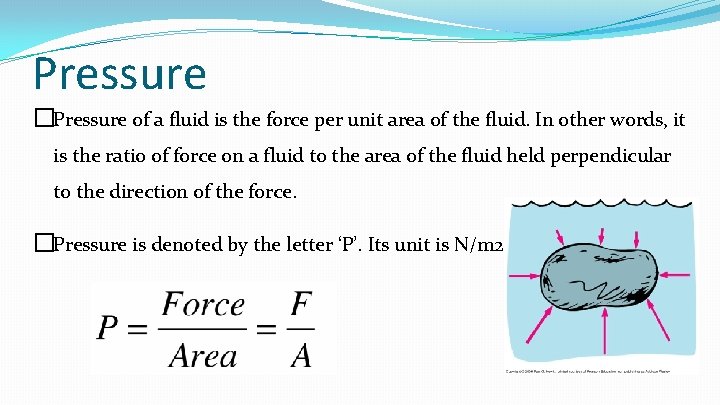
Pressure �Pressure of a fluid is the force per unit area of the fluid. In other words, it is the ratio of force on a fluid to the area of the fluid held perpendicular to the direction of the force. �Pressure is denoted by the letter ‘P’. Its unit is N/m 2.

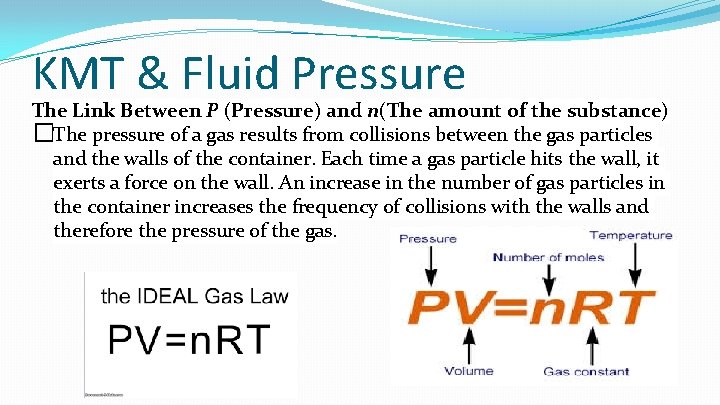
KMT & Fluid Pressure The Link Between P (Pressure) and n(The amount of the substance) �The pressure of a gas results from collisions between the gas particles and the walls of the container. Each time a gas particle hits the wall, it exerts a force on the wall. An increase in the number of gas particles in the container increases the frequency of collisions with the walls and therefore the pressure of the gas.

Viscosity � Viscosity, m, is the property of a fluid, due to cohesion and interaction between molecules, which offers resistance to sheer deformation. Different fluids deform at different rates under the same shear stress. Fluid with a high viscosity such as syrup, deforms more slowly than fluid with a low viscosity such as water. What does that mean? It means that viscosity is the measure of how capable it is of flowing. � In a liquid, viscosity decreases with increase in temperature. In a gas, viscosity increases with increase in temperature. � Viscosity is also known as the resistance to flow.


Newtonian Fluids � Many people have heard of Sir Isaac Newton. He is famous for developing many scientific theories in mathematics and physics. Newton described how ‘normal’ liquids or fluids behave, and he observed that they have a constant viscosity (flow). This means that their flow behaviour or viscosity only changes with changes in temperature or pressure. For example, water freezes and turns into a solid at 0˚C and turns into a gas at 100˚C. Within this temperature range, water behaves like a ‘normal’ liquid with constant viscosity. � Typically, liquids take on the shape of the container they are poured into. We call these ‘normal liquids’ Newtonian fluids. � Some examples of Newtonian fluids include water, air, motor oil, and organic solvents. For those fluids viscosity is only dependent on temperature.

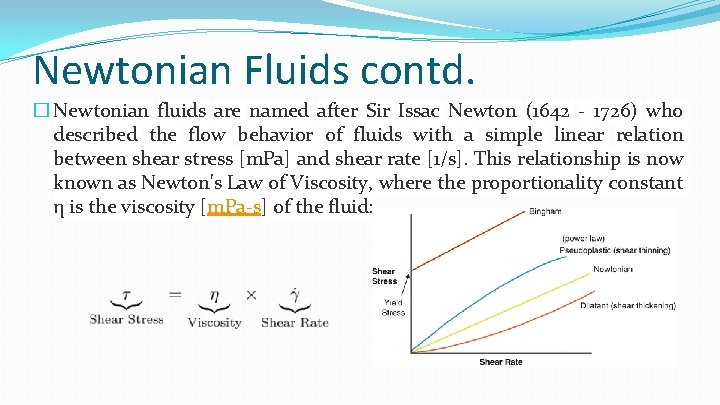
Newtonian Fluids contd. � Newtonian fluids are named after Sir Issac Newton (1642 - 1726) who described the flow behavior of fluids with a simple linear relation between shear stress [m. Pa] and shear rate [1/s]. This relationship is now known as Newton's Law of Viscosity, where the proportionality constant η is the viscosity [m. Pa-s] of the fluid:

Shear Stress & Shear Rate � Shear stress is defined as a force per unit area, acting parallel to an infinitesimal surface element. Shear stress is primarily caused by friction between fluid particles, due to fluid viscosity. � Shear rate is defined is the rate at which a progressive shearing deformation is applied to some material. The rate of the change in a material's volume in response to stress. � Each unique material has its own behavior when subjected to flow, deformation or stress. Depending on their viscosity behavior as a function of shear rate and stress, fluids are characterized as Newtonian or non-Newtonian.

Non Newtonian Fluids � But some fluids don’t follow this rule. We call these ‘strange liquids’ non. Newtonian fluids. � Non-Newtonian fluids change their viscosity or flow behaviour under stress. If you apply a force to such fluids (say you hit, shake or jump on them), the sudden application of stress can cause them to get thicker and act like a solid, or in some cases it results in the opposite behaviour and they may get runnier than they were before. Remove the stress (let them sit still or only move them slowly) and they will return to their earlier state.

Non Newtonian Fluids contd. � Say you want to get some ketchup out of the bottle. You know there is some in there, but when you turn the bottle upside down, nothing comes out. So what do you do? You shake or hit the bottle. This causes the ketchup to become more liquid and you can easily squirt some out. In this case, the ketchup’s viscosity decreases and it gets runnier with applied stress.

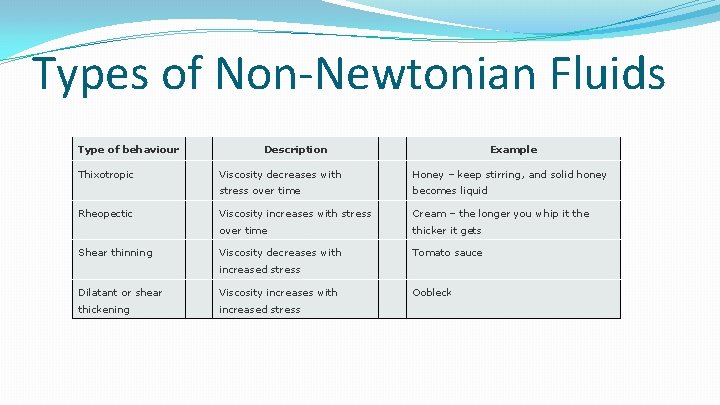
Types of Non-Newtonian Fluids Type of behaviour Thixotropic Rheopectic Shear thinning Description Example Viscosity decreases with Honey – keep stirring, and solid honey stress over time becomes liquid Viscosity increases with stress Cream – the longer you whip it the over time thicker it gets Viscosity decreases with Tomato sauce increased stress Dilatant or shear Viscosity increases with thickening increased stress Oobleck

Oobleck - extra credit option � Oobleck is a mixture of cornflour and water (similar to uncooked custard) named after a substance in a Dr Seuss book. This liquid is a runny goo until you apply stress to it, and then it suddenly acts like a solid. You can hit a bowlful with a hammer, and instead of splashing everywhere, the particles lock together. You can roll it into a solid ball in your hand, but if you stop moving it, it reverts to liquid and oozes out through your fingers. In this case, the oobleck’s viscosity or resistance to flow increases with applied stress. Recipe: - 1. 5 -2 cups corn starch - 1 cup water - Food coloring

Other Properties of Fluids Just FYI

Specific Volume �Specific volume is the volume of a fluid (V) occupied per unit mass (m). It is the reciprocal of density. �Specific volume is denoted by the symbol ‘v’. Its unit is m 3/kg.

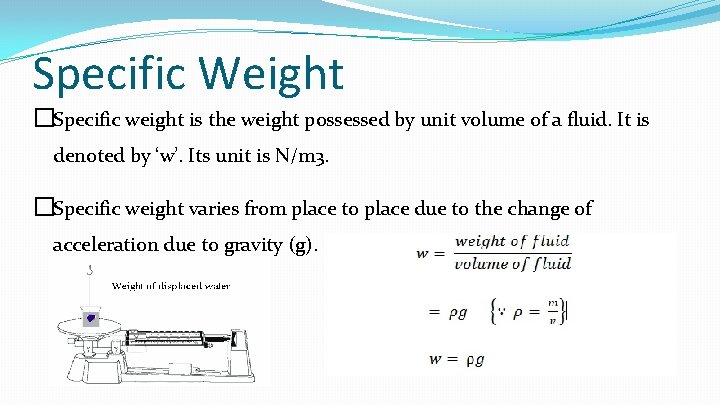
Specific Weight �Specific weight is the weight possessed by unit volume of a fluid. It is denoted by ‘w’. Its unit is N/m 3. �Specific weight varies from place to place due to the change of acceleration due to gravity (g).

Specific Gravity �Specific gravity is the ratio of specific weight of the given fluid to the specific weight of standard fluid. It is denoted by the letter ‘S’. It has no unit.
 Buoyancyability
Buoyancyability Volumetric properties of pure fluids
Volumetric properties of pure fluids How does water go through your body
How does water go through your body Mild ascites symptoms
Mild ascites symptoms P1-p2
P1-p2 Hypoosmotic
Hypoosmotic Fluid statics deals with
Fluid statics deals with Fluid kinematics
Fluid kinematics Intracellular fluid and extracellular fluid examples
Intracellular fluid and extracellular fluid examples Extracellular fluid and interstitial fluid
Extracellular fluid and interstitial fluid Is synovial fluid extracellular fluid
Is synovial fluid extracellular fluid Air properties calculator
Air properties calculator Difference between intensive and extensive properties
Difference between intensive and extensive properties Chemical property definition
Chemical property definition Eparing
Eparing Crystalloid solution example
Crystalloid solution example Power law fluids
Power law fluids Fluids physics problems and solutions
Fluids physics problems and solutions Static fluids
Static fluids Fluid flow classification
Fluid flow classification