Physical Science Unit 6 Crash Course Chapter 7






- Slides: 13

Physical Science Unit 6 Crash Course Chapter 7, 8, and 10 (Chemical Reactions, Solutions, and Nuclear Chemistry)

Chapter 7 – Chemical Reactions Reactants Products The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. Balance If hydrogen and oxygen last. you have an odd number of atoms on one side, you can multiply by 2 to make it even.

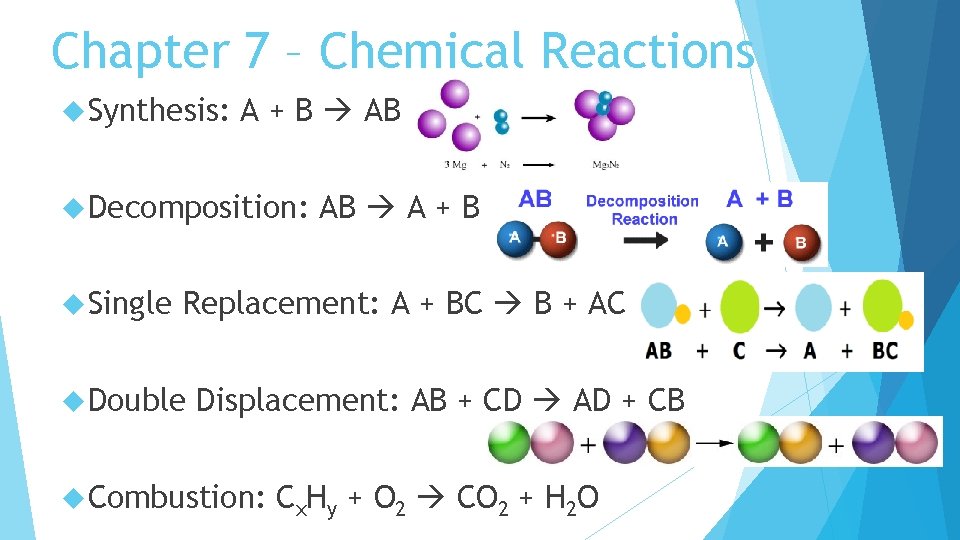
Chapter 7 – Chemical Reactions Synthesis: A + B AB Decomposition: Single AB A + B Replacement: A + BC B + AC Double Displacement: AB + CD AD + CB Combustion: Cx. Hy + O 2 CO 2 + H 2 O

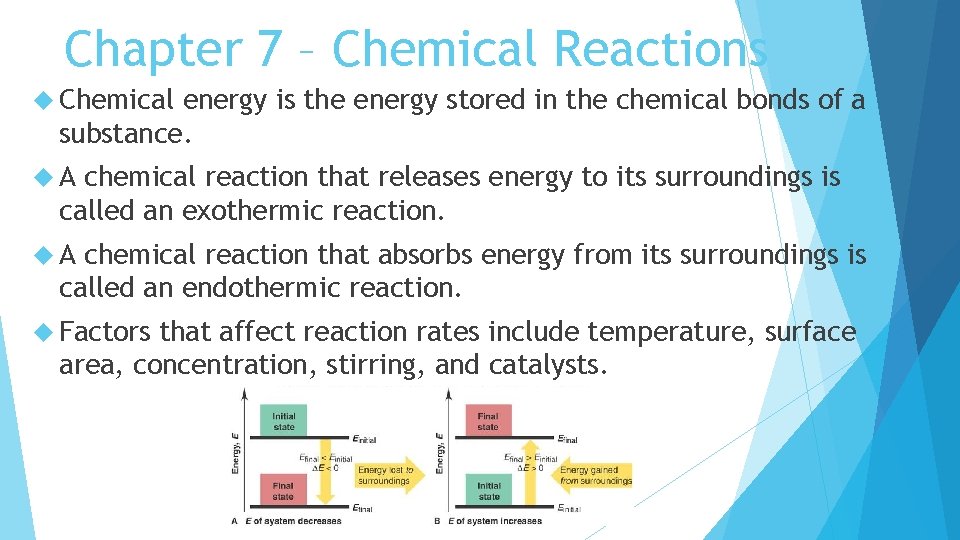
Chapter 7 – Chemical Reactions Chemical energy is the energy stored in the chemical bonds of a substance. A chemical reaction that releases energy to its surroundings is called an exothermic reaction. A chemical reaction that absorbs energy from its surroundings is called an endothermic reaction. Factors that affect reaction rates include temperature, surface area, concentration, stirring, and catalysts.

Chapter 8 – Solutions Every solution has two types of components. A solute is a substance that is dissolved in a solution. The substance in which the solute dissolves is called the solvent. Three physical properties of a solution that can differ from those of its solute and solvent are conductivity, freezing point, and boiling point. A solute will lower the freezing point of a solvent. A solute can also raise the boiling point of a solvent.

Chapter 8 – Solutions The solution process can be described as exothermic or endothermic. Factors that affect the rate of dissolving include surface area, stirring, and temperature. The maximum amount of solute that dissolve in a given amount of solvent at a constant temperature is called solubility.

Chapter 8 – Solutions A solution that has less than the maximum amount of solute that can be dissolved is called an unsaturated solution. A saturated solution is one that contains as much solute as the solvent can hold at a given temperature. A supersaturated solution is one that contains more solute than it can normally hold at a given temperature.

Chapter 8 – Solutions In general, the solubility of solids increases as the solvent temperature increases. The solubility of gases usually decreases as the temperature of the solvent increases. Increasing the pressure of a gas increases its solubility in a liquid. An acid is a compound that produces hydronium ions (H 3 O+) when dissolved in water. Some general properties of acids include sour taste, reactivity with metals, and ability to produce color changes in indicators.

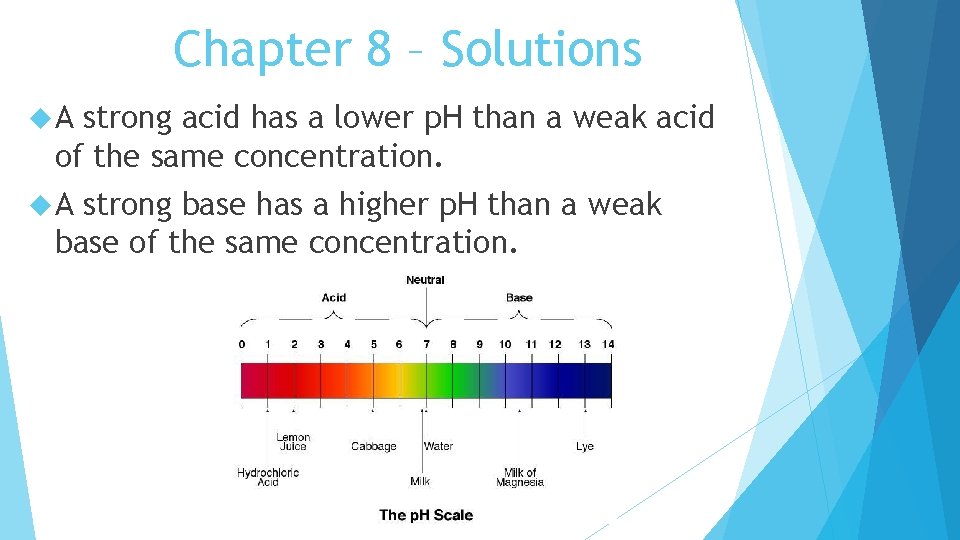
Chapter 8 – Solutions A base is a compound that produces hydroxide ions (OH-) when dissolved in water. Some general properties of bases include bitter taste, slippery feel, and ability to produce color changes in indicators. Chemists use a number scale called the p. H scale that ranges from 0 to 14 to describe the concentration of hydronium ions (H 3 O+) in a solution.

Chapter 8 – Solutions A strong acid has a lower p. H than a weak acid of the same concentration. A strong base has a higher p. H than a weak base of the same concentration.

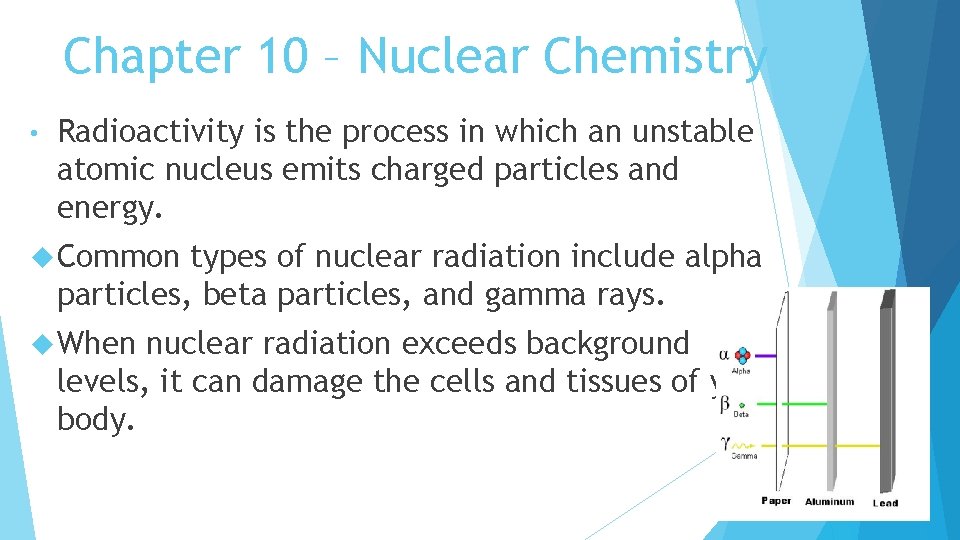
Chapter 10 – Nuclear Chemistry • Radioactivity is the process in which an unstable atomic nucleus emits charged particles and energy. Common types of nuclear radiation include alpha particles, beta particles, and gamma rays. When nuclear radiation exceeds background levels, it can damage the cells and tissues of your body.

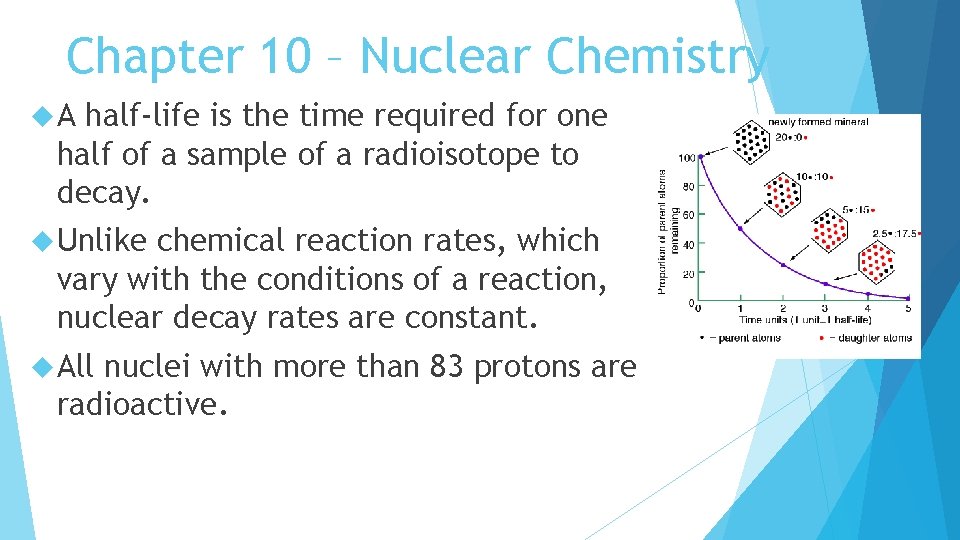
Chapter 10 – Nuclear Chemistry A half-life is the time required for one half of a sample of a radioisotope to decay. Unlike chemical reaction rates, which vary with the conditions of a reaction, nuclear decay rates are constant. All nuclei with more than 83 protons are radioactive.

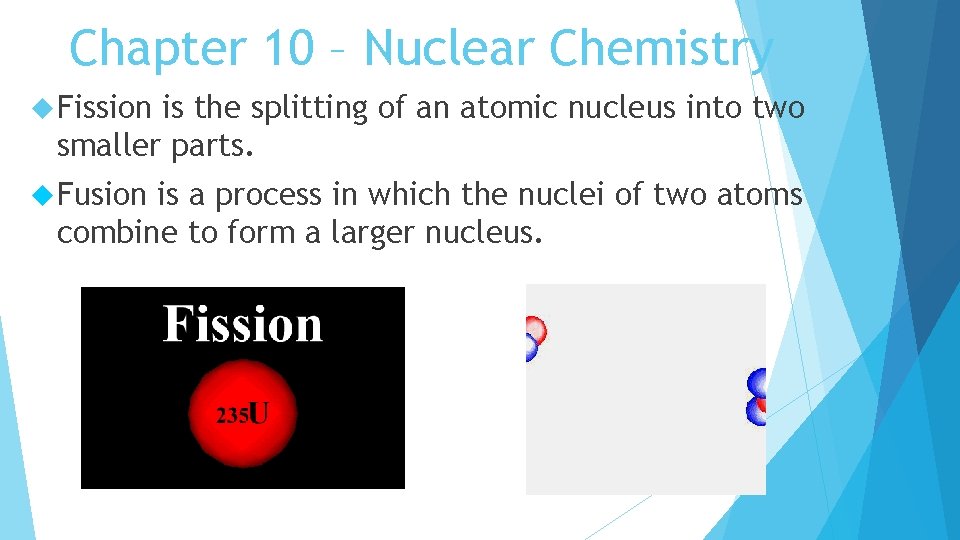
Chapter 10 – Nuclear Chemistry Fission is the splitting of an atomic nucleus into two smaller parts. Fusion is a process in which the nuclei of two atoms combine to form a larger nucleus.