Phyllosilicates Talc and Pyrophyllite Talc Mg 3 Si




- Slides: 12

Phyllosilicates: Talc and Pyrophyllite Talc Mg 3 Si 4 O 10(OH)2 Pyrophyllite Al 2 Si 4 O 10(OH)2

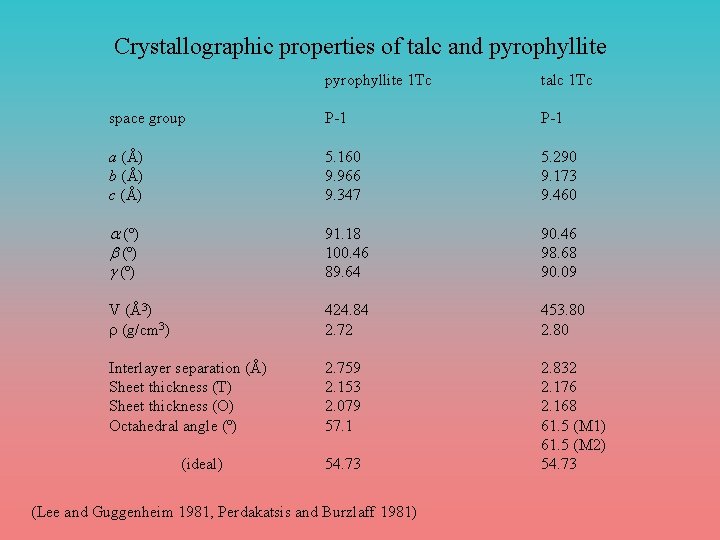
Crystallographic properties of talc and pyrophyllite 1 Tc talc 1 Tc space group P-1 a (Å) b (Å) c (Å) 5. 160 9. 966 9. 347 5. 290 9. 173 9. 460 a (º) b (º) g (º) 91. 18 100. 46 89. 64 90. 46 98. 68 90. 09 V (Å3) r (g/cm 3) 424. 84 2. 72 453. 80 2. 80 Interlayer separation (Å) Sheet thickness (T) Sheet thickness (O) Octahedral angle (º) 2. 759 2. 153 2. 079 57. 1 2. 832 2. 176 2. 168 61. 5 (M 1) 61. 5 (M 2) 54. 73 (ideal) 54. 73 (Lee and Guggenheim 1981, Perdakatsis and Burzlaff 1981)

c-axis projection of T-O-T sheet in talc. Light green - Mg 1, Dark Green - Mg 2, Blue - Si, Red - O, small spheres - H (Perdikatsis and Burzlaff 1981)

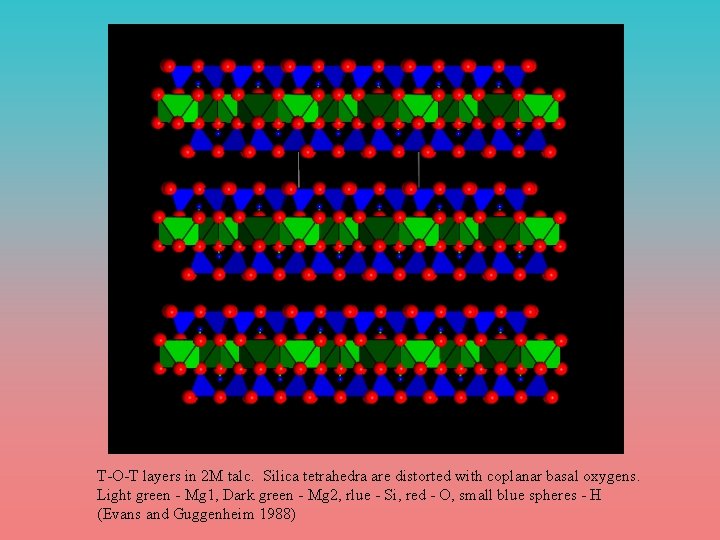
T-O-T layers in 2 M talc. Silica tetrahedra are distorted with coplanar basal oxygens. Light green - Mg 1, Dark green - Mg 2, rlue - Si, red - O, small blue spheres - H (Evans and Guggenheim 1988)

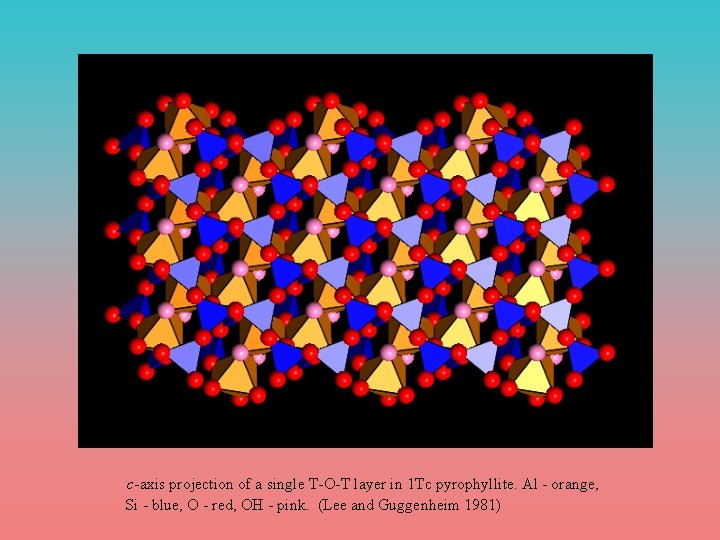
c-axis projection of a single T-O-T layer in 1 Tc pyrophyllite. Al - orange, Si - blue, O - red, OH - pink. (Lee and Guggenheim 1981)

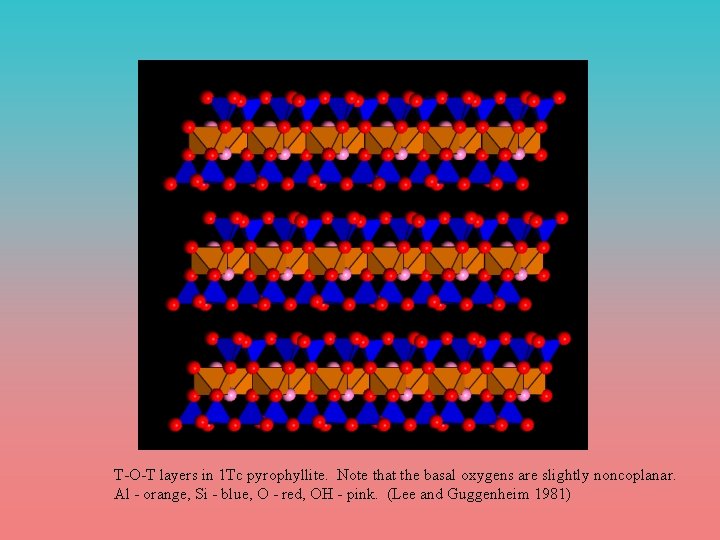
T-O-T layers in 1 Tc pyrophyllite. Note that the basal oxygens are slightly noncoplanar. Al - orange, Si - blue, O - red, OH - pink. (Lee and Guggenheim 1981)

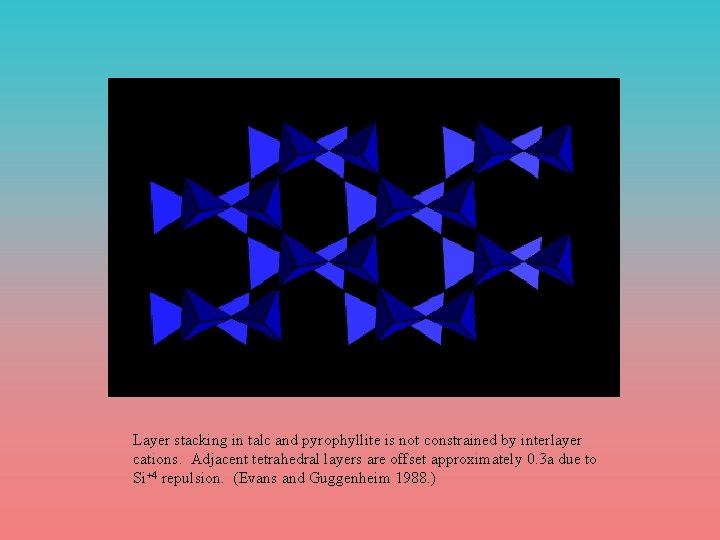
Layer Stacking • Not constrained by intelayer cations (as in micas) or hydrogen bonding. • Empty cation sites between layers are large and irregular, therefore the structure is distinct from a cation-deficient mica • Stacking disorder is ubiquitous, therefore it is not worthwhile to differentiate between polytypes generally between crystals • T-O-T layers are shifted by approximately 0. 3 a to minimize Si - Si repulsion • Common polytypes are 2 M for talc and 1 Tc for pyrophyllite, however 22 pyrophyllite polytypes and 10 talc polytypes are known, only some of which can be identified by x-ray analysis (Durovic and Weiss 1979, Bailey 1984)

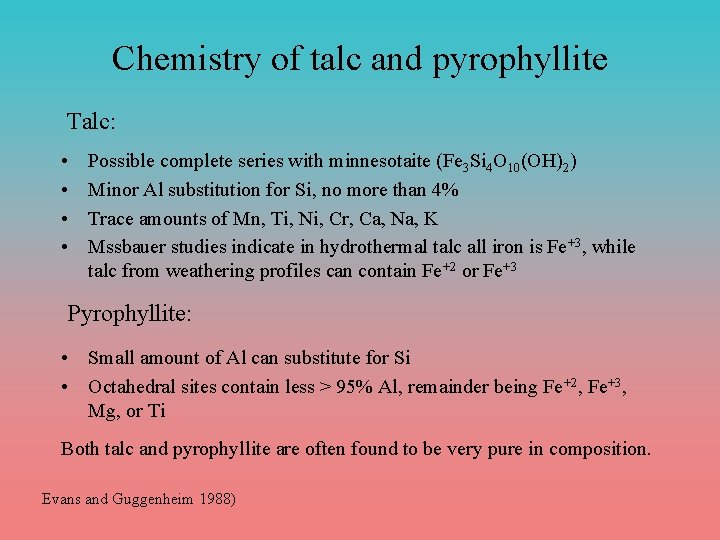
Chemistry of talc and pyrophyllite Talc: • • Possible complete series with minnesotaite (Fe 3 Si 4 O 10(OH)2) Minor Al substitution for Si, no more than 4% Trace amounts of Mn, Ti, Ni, Cr, Ca, Na, K Mssbauer studies indicate in hydrothermal talc all iron is Fe+3, while talc from weathering profiles can contain Fe+2 or Fe+3 Pyrophyllite: • Small amount of Al can substitute for Si • Octahedral sites contain less > 95% Al, remainder being Fe+2, Fe+3, Mg, or Ti Both talc and pyrophyllite are often found to be very pure in composition. Evans and Guggenheim 1988)

Layer stacking in talc and pyrophyllite is not constrained by interlayer cations. Adjacent tetrahedral layers are offset approximately 0. 3 a due to Si+4 repulsion. (Evans and Guggenheim 1988. )

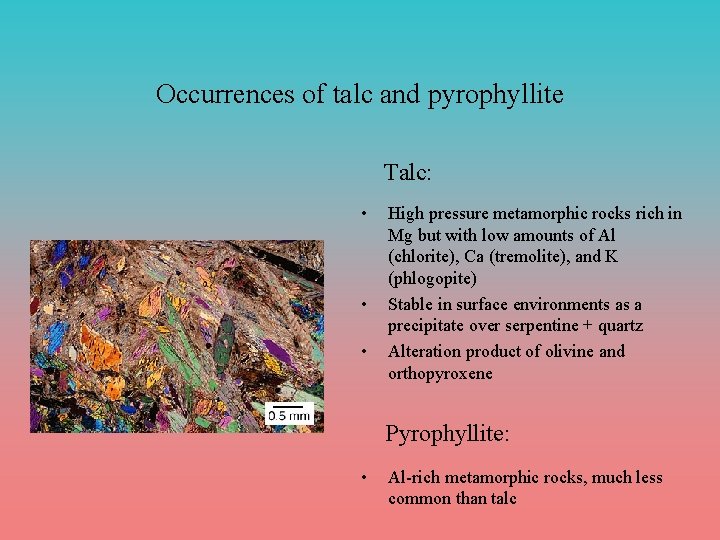
Occurrences of talc and pyrophyllite Talc: • • • High pressure metamorphic rocks rich in Mg but with low amounts of Al (chlorite), Ca (tremolite), and K (phlogopite) Stable in surface environments as a precipitate over serpentine + quartz Alteration product of olivine and orthopyroxene Pyrophyllite: • Al-rich metamorphic rocks, much less common than talc

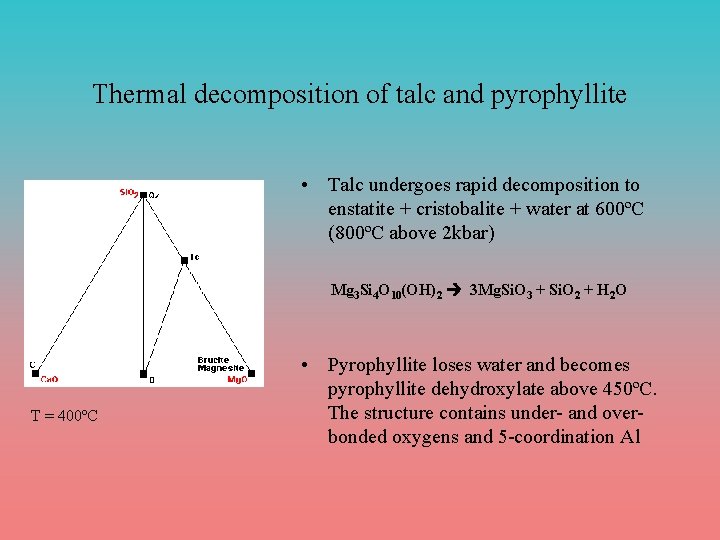
Thermal decomposition of talc and pyrophyllite • Talc undergoes rapid decomposition to enstatite + cristobalite + water at 600ºC (800ºC above 2 kbar) Mg 3 Si 4 O 10(OH)2 à 3 Mg. Si. O 3 + Si. O 2 + H 2 O T = 400ºC • Pyrophyllite loses water and becomes pyrophyllite dehydroxylate above 450ºC. The structure contains under- and overbonded oxygens and 5 -coordination Al

Uses of talc and pyrophyllite • • • Used in the manufacture of ceramics As a filler material in plastic, rubber, paint, insecticides, paper, and other materials Made into acid and heat resistant laboratory countertops Cosmetics and personal products Used as an ornamental stone for carvings