Pharmacogenetics for Genes Associated with AgeRelated Macular Degeneration
- Slides: 10

Pharmacogenetics for Genes Associated with Age-Related Macular Degeneration in the Comparison of AMD Treatments Trials (CATT) Hagstrom SA, Ying G-S, Pauer GJT, Sturgill-Short GM, Huang J, Callanan DG, Kim IK, Klein ML, Maguire MG, MG Martin DF for the CATT Research Group Available through http: //www. med. upenn. edu/cpob/publications_main. shtml Supported by Cooperative Agreements from the National Eye Institute, National Institutes of Health, DHHS 1

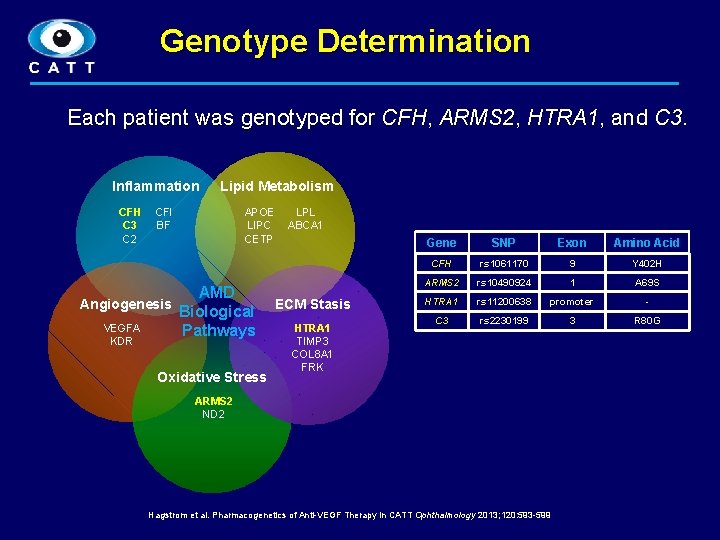
Genotype Determination Each patient was genotyped for CFH, ARMS 2, HTRA 1, and C 3. Inflammation CFH C 3 C 2 Lipid Metabolism CFI BF APOE LIPC CETP AMD Angiogenesis Biological VEGFA Pathways KDR Oxidative Stress LPL ABCA 1 ECM Stasis HTRA 1 TIMP 3 COL 8 A 1 FRK Gene SNP Exon Amino Acid CFH rs 1061170 9 Y 402 H ARMS 2 rs 10490924 1 A 69 S HTRA 1 rs 11200638 promoter - C 3 rs 2230199 3 R 80 G ARMS 2 ND 2 Hagstrom et al. Pharmacogenetics of Anti-VEGF Therapy in CATT Ophthalmology 2013; 120: 593 -599

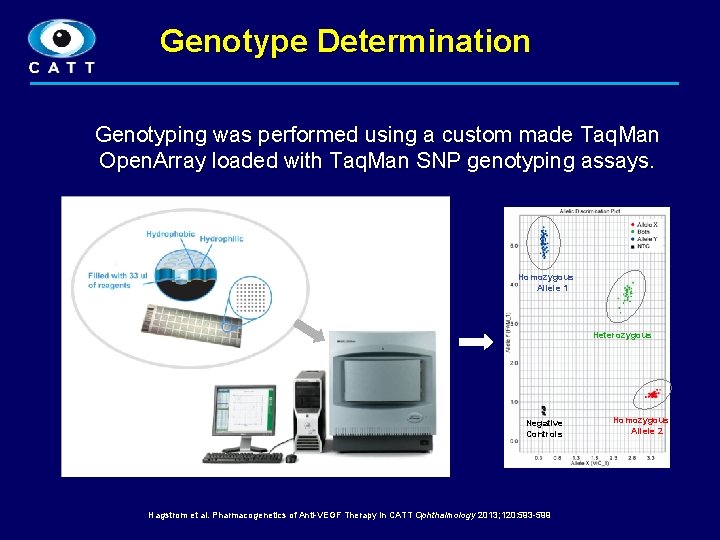
Genotype Determination Genotyping was performed using a custom made Taq. Man Open. Array loaded with Taq. Man SNP genotyping assays. Homozygous Allele 1 Heterozygous Negative Controls Hagstrom et al. Pharmacogenetics of Anti-VEGF Therapy in CATT Ophthalmology 2013; 120: 593 -599 Homozygous Allele 2

Outcome Variables All outcomes were determined following standardized protocols. VA assessed using e. ETDRS testing OCT outcomes determined by independent OCT Reading Center FA and photographic outcomes determined by independent Fundus Photographic Reading Center Hagstrom et al. Pharmacogenetics of Anti-VEGF Therapy in CATT Ophthalmology 2013; 120: 593 -599

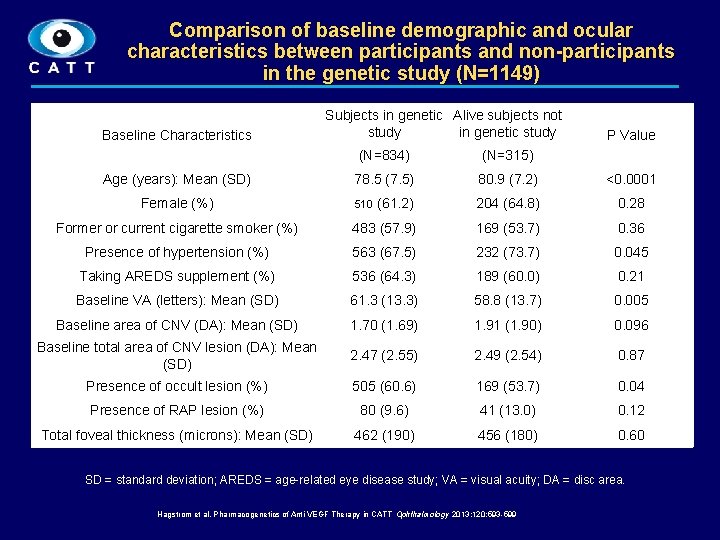
Comparison of baseline demographic and ocular characteristics between participants and non-participants in the genetic study (N=1149) Baseline Characteristics Subjects in genetic Alive subjects not study in genetic study P Value (N=834) (N=315) 78. 5 (7. 5) 80. 9 (7. 2) <0. 0001 (61. 2) 204 (64. 8) 0. 28 Former or current cigarette smoker (%) 483 (57. 9) 169 (53. 7) 0. 36 Presence of hypertension (%) 563 (67. 5) 232 (73. 7) 0. 045 Taking AREDS supplement (%) 536 (64. 3) 189 (60. 0) 0. 21 Baseline VA (letters): Mean (SD) 61. 3 (13. 3) 58. 8 (13. 7) 0. 005 Baseline area of CNV (DA): Mean (SD) 1. 70 (1. 69) 1. 91 (1. 90) 0. 096 Baseline total area of CNV lesion (DA): Mean (SD) 2. 47 (2. 55) 2. 49 (2. 54) 0. 87 Presence of occult lesion (%) 505 (60. 6) 169 (53. 7) 0. 04 Presence of RAP lesion (%) 80 (9. 6) 41 (13. 0) 0. 12 Total foveal thickness (microns): Mean (SD) 462 (190) 456 (180) 0. 60 Age (years): Mean (SD) Female (%) 510 SD = standard deviation; AREDS = age-related eye disease study; VA = visual acuity; DA = disc area. Hagstrom et al. Pharmacogenetics of Anti-VEGF Therapy in CATT Ophthalmology 2013; 120: 593 -599

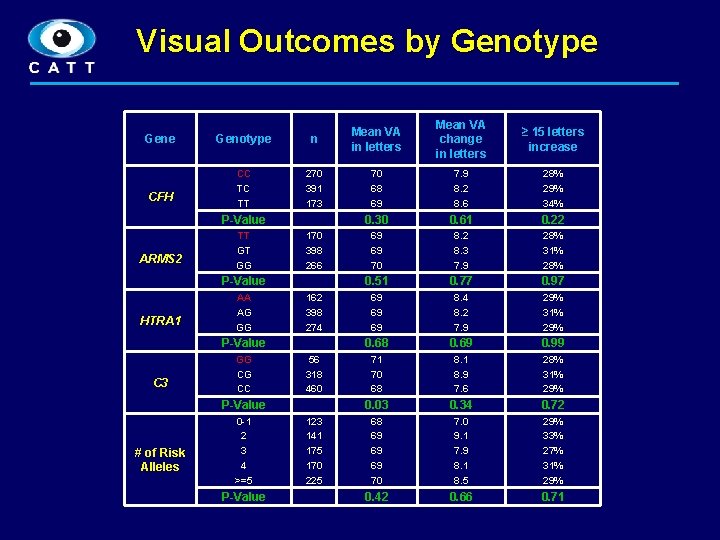
Visual Outcomes by Genotype Gene CFH Genotype n Mean VA in letters Mean VA change in letters ≥ 15 letters increase CC TC TT 270 391 173 70 68 69 7. 9 8. 2 8. 6 28% 29% 34% 0. 30 0. 61 0. 22 69 69 70 8. 2 8. 3 7. 9 28% 31% 28% 0. 51 0. 77 0. 97 69 69 69 8. 4 8. 2 7. 9 29% 31% 29% 0. 68 0. 69 0. 99 71 70 68 8. 1 8. 9 7. 6 28% 31% 29% 0. 03 0. 34 0. 72 68 69 69 69 70 7. 0 9. 1 7. 9 8. 1 8. 5 29% 33% 27% 31% 29% 0. 42 0. 66 0. 71 P-Value ARMS 2 TT GT GG 170 398 266 P-Value HTRA 1 AA AG GG 162 398 274 P-Value C 3 GG CG CC 56 318 460 P-Value # of Risk Alleles 0 -1 2 3 4 >=5 P-Value 123 141 175 170 225 6

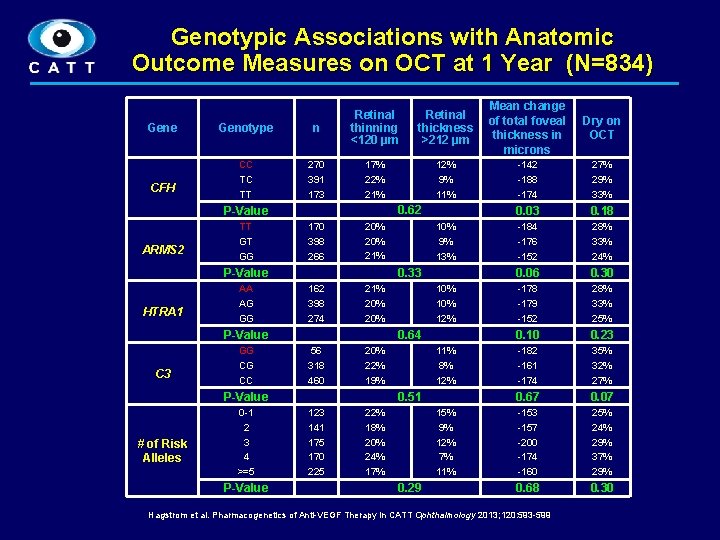
Genotypic Associations with Anatomic Outcome Measures on OCT at 1 Year (N=834) Gene CFH Genotype n Retinal thinning <120 µm CC TC TT 270 391 173 17% 22% 21% ARMS 2 170 398 266 HTRA 1 C 3 162 398 274 21% 20% # of Risk Alleles P-Value 10% 12% 0. 64 56 318 460 20% 22% 19% 123 141 175 170 225 22% 18% 20% 24% 17% P-Value 0 -1 2 3 4 >=5 10% 9% 13% 0. 33 P-Value GG CG CC 11% 20% 21% P-Value AA AG GG 12% 9% 0. 62 P-Value TT GT GG Retinal thickness >212 µm 11% 8% 12% 0. 51 15% 9% 12% 7% 11% 0. 29 Mean change of total foveal thickness in microns Dry on OCT -142 -188 -174 27% 29% 33% 0. 03 0. 18 -184 -176 -152 28% 33% 24% 0. 06 0. 30 -178 -179 -152 28% 33% 25% 0. 10 0. 23 -182 -161 -174 35% 32% 27% 0. 67 0. 07 -153 -157 -200 -174 -160 25% 24% 29% 37% 29% 0. 68 0. 30 Hagstrom et al. Pharmacogenetics of Anti-VEGF Therapy in CATT Ophthalmology 2013; 120: 593 -599 7

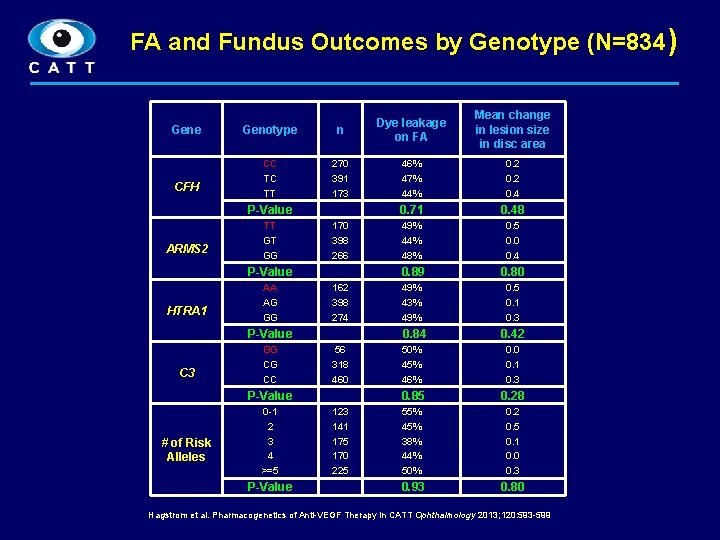
FA and Fundus Outcomes by Genotype (N=834) Gene CFH Genotype n CC TC TT 270 391 173 46% 47% 44% 0. 2 0. 4 0. 71 0. 48 49% 44% 48% 0. 5 0. 0 0. 4 0. 89 0. 80 49% 43% 49% 0. 5 0. 1 0. 3 P-Value ARMS 2 TT GT GG 170 398 266 P-Value HTRA 1 AA AG GG 162 398 274 P-Value C 3 GG CG CC 0. 84 56 318 460 P-Value # of Risk Alleles Mean change in lesion size in disc area Dye leakage on FA 0 -1 2 3 4 >=5 P-Value 123 141 175 170 225 0. 42 50% 45% 46% 0. 0 0. 1 0. 3 0. 85 0. 28 55% 45% 38% 44% 50% 0. 2 0. 5 0. 1 0. 0 0. 3 0. 93 0. 80 Hagstrom et al. Pharmacogenetics of Anti-VEGF Therapy in CATT Ophthalmology 2013; 120: 593 -599 8

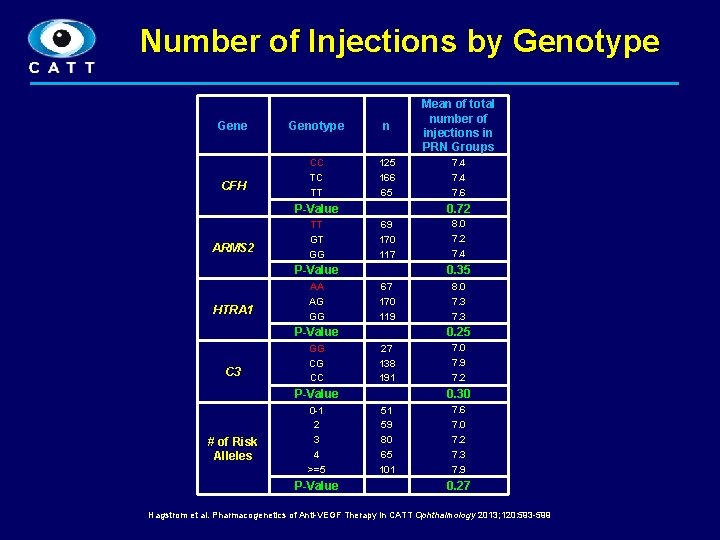
Number of Injections by Genotype Gene CFH Genotype n Mean of total number of injections in PRN Groups CC TC TT 125 166 65 7. 4 7. 6 P-Value ARMS 2 TT GT GG 0. 72 69 170 117 0. 35 P-Value HTRA 1 AA AG GG 67 170 119 C 3 27 138 191 P-Value # of Risk Alleles 0 -1 2 3 4 >=5 P-Value 8. 0 7. 3 0. 25 P-Value GG CG CC 8. 0 7. 2 7. 4 7. 0 7. 9 7. 2 0. 30 51 59 80 65 101 7. 6 7. 0 7. 2 7. 3 7. 9 0. 27 Hagstrom et al. Pharmacogenetics of Anti-VEGF Therapy in CATT Ophthalmology 2013; 120: 593 -599 9

Conclusions Largest pharmacogenetic analysis exploring the relationship between genotypes and response to anti-VEGF therapy with >90% power to detect a 1 line difference between the highest risk and lowest risk groups. The relationship between genotype and response to therapy did not vary by drug or dosing regimen. Although specific risk alleles for CFH, ARMS 2, HTRA 1 and C 3 may predict the development of AMD, they did not predict response to anti-VEGF therapy. Further studies targeting SNPs in additional biological pathways are ongoing. Hagstrom et al. Pharmacogenetics of Anti-VEGF Therapy in CATT Ophthalmology 2013; 120: 593 -599 10