Patient Followup Streamlining Data Collection ACST2 Overview First









- Slides: 16

Patient Follow-up & Streamlining Data Collection

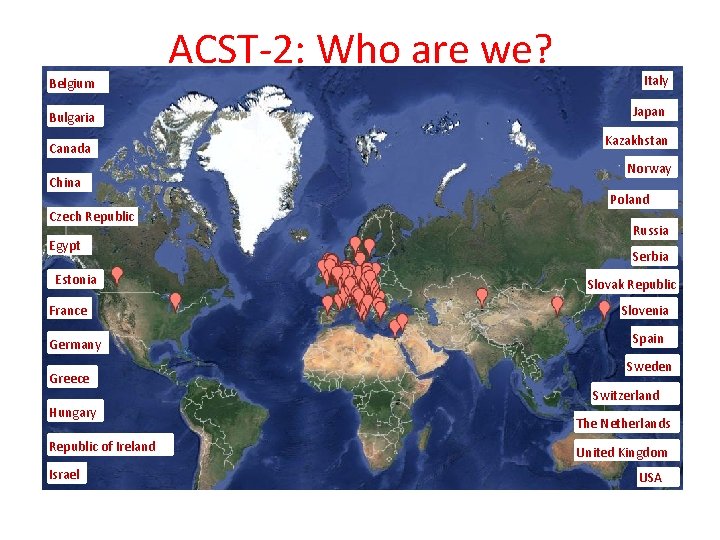
ACST-2: Overview • First patient randomised: 15/01/2008 • Patients are now in their 6 th year of follow up • 93 Active Centres from 27 countries

Belgium Bulgaria Canada China Czech Republic Egypt Estonia France Germany Greece Hungary Republic of Ireland Israel ACST-2: Who are we? Italy Japan Kazakhstan Norway Poland Russia Serbia Slovak Republic Slovenia Spain Sweden Switzerland The Netherlands United Kingdom USA

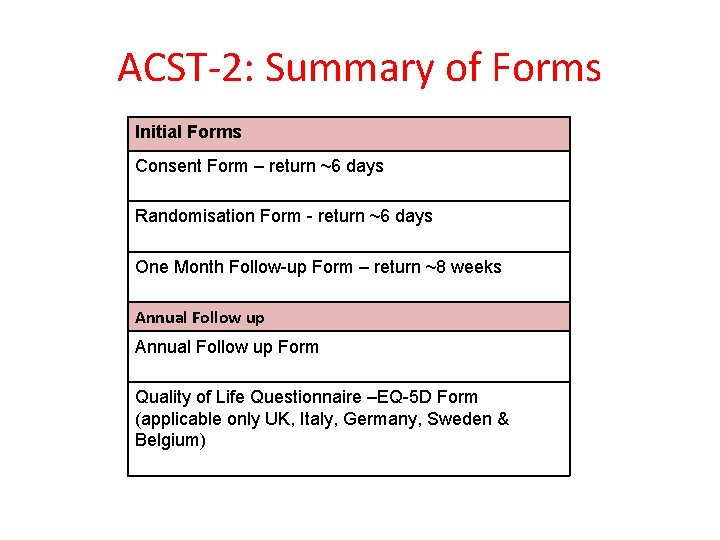
ACST-2: Summary of Forms Initial Forms Consent Form – return ~6 days Randomisation Form - return ~6 days One Month Follow-up Form – return ~8 weeks Annual Follow up Form Quality of Life Questionnaire –EQ-5 D Form (applicable only UK, Italy, Germany, Sweden & Belgium)

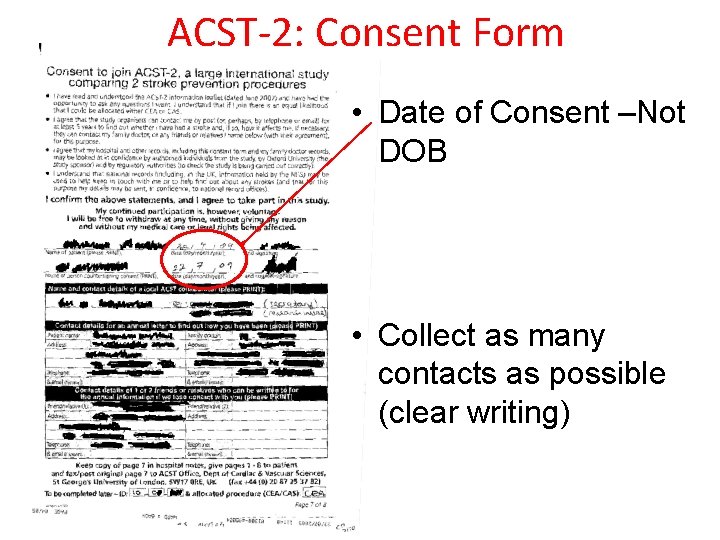
ACST-2: Consent Form • Date of Consent –Not DOB • Collect as many contacts as possible (clear writing)

ACST-2: Randomisation Form If patient was symptomatic in the ipsilateral territory please confirm this was more than 6 months ago Please do not use √ or X

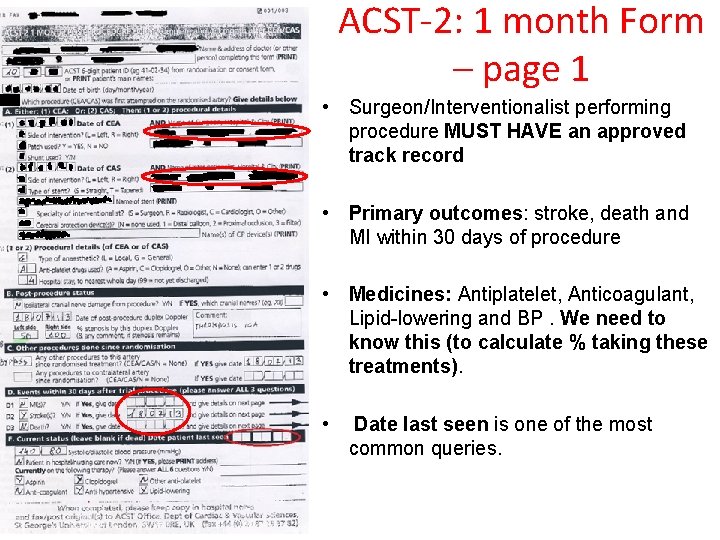
ACST-2: 1 month Form – page 1 • Surgeon/Interventionalist performing procedure MUST HAVE an approved track record • Primary outcomes: stroke, death and MI within 30 days of procedure • Medicines: Antiplatelet, Anticoagulant, Lipid-lowering and BP. We need to know this (to calculate % taking these treatments). • Date last seen is one of the most common queries.

ACST-2: 1 month Form- page 2 • Details of major events • Comments section; for crossovers, procedure not yet done or anything else you need to tell us

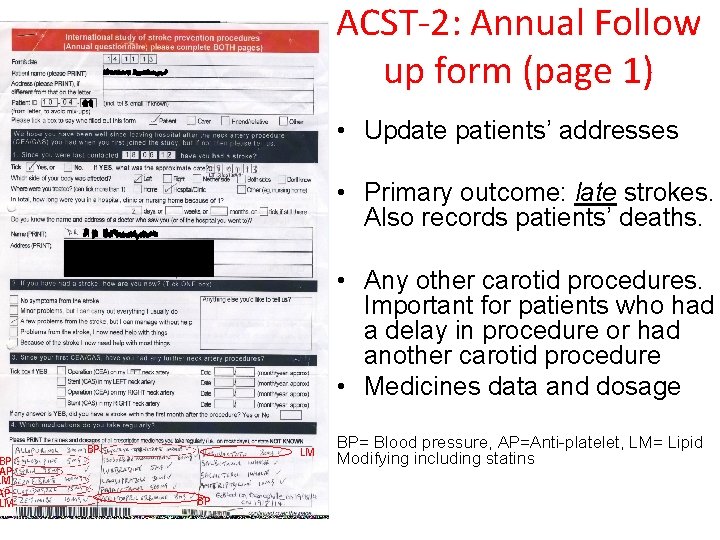
BP AP LM ACST-2: Annual Follow up form (page 1) • Update patients’ addresses • Primary outcome: late strokes. Also records patients’ deaths. • Any other carotid procedures. Important for patients who had a delay in procedure or had another carotid procedure • Medicines data and dosage BP LM BP BP= Blood pressure, AP=Anti-platelet, LM= Lipid Modifying including statins

ACST-2: Annual Follow up form (page 2) • Captures any changes in contact details for family doctor & relatives. • Your patient has opportunity to ask questions! • Date of completion is important for following year’s form

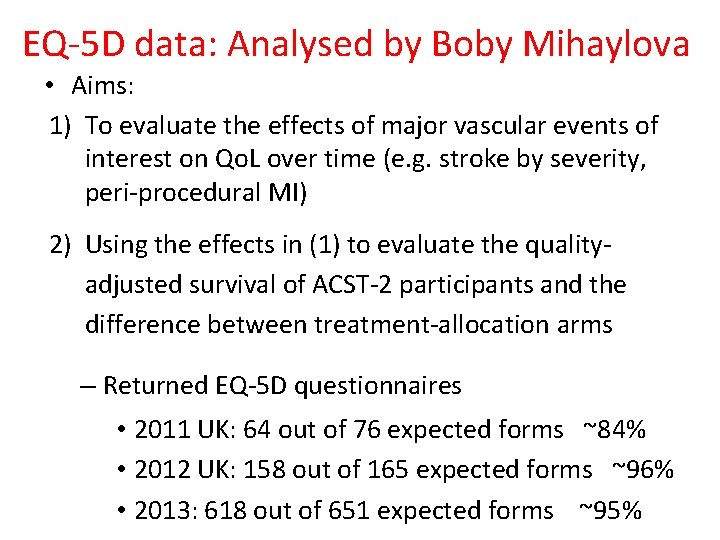
EQ-5 D data: Analysed by Boby Mihaylova • Aims: 1) To evaluate the effects of major vascular events of interest on Qo. L over time (e. g. stroke by severity, peri-procedural MI) 2) Using the effects in (1) to evaluate the qualityadjusted survival of ACST-2 participants and the difference between treatment-allocation arms – Returned EQ-5 D questionnaires • 2011 UK: 64 out of 76 expected forms ~84% • 2012 UK: 158 out of 165 expected forms ~96% • 2013: 618 out of 651 expected forms ~95%

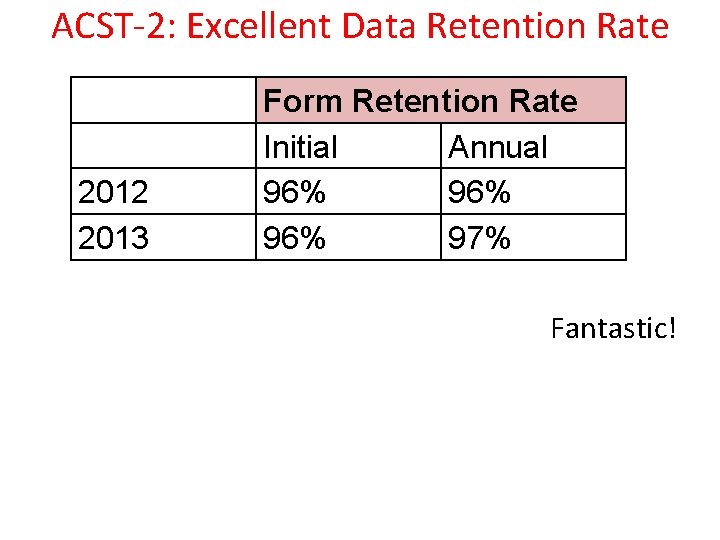
ACST-2: Excellent Data Retention Rate 2012 2013 Form Retention Rate Initial Annual 96% 96% 97% Fantastic!

What you may not know about ACST-2 • Every year (April/May) we report to the Data Monitoring Committee. • We report all major events, any missing forms, procedures not done, crossovers and any other important details from each centre.

What you may not know about ACST-2 last year …. • You helped us answer over 2000 queries – thank you! • Over 250 possible ME queries came to us (Most are not ME’s but this is good) • Annual letters – sent for any procedure not done or to request information when your patient’s Annual Form has said they have had a procedure • We only require a copy of the forms, the original can be kept in your patient files

Plans for the next year • 2014 Aim - to receive at least 98% of Annual Follow up forms • Please complete our short questionnaire; this will allow us to find out how you prefer to receive queries. • Improve our training for centres – in order to reduce numbers of queries • Reduce the major event turn around time • Maintain your excellent retention rate for all forms

Thank you • The great data we already have in ACST-2 would not be possible without all of you – our collaborators! • Every form, item of data and email response counts!!