OUTCOMES OF ORAL CARE KITS ON ORAL HEALTH






- Slides: 17

OUTCOMES OF ORAL CARE KITS ON ORAL HEALTH FOR PATIENTS AT RISK FOR DEVELOPING ORAL MUCOSITIS SHAN LI & HABIB KOUSSE

BACKGROUND • Oral mucositis describes inflammation of oral mucosa resulting from chemotherapeutic agents or ionizing radiation. • It is the most distressing side effect from treatment of cancer by chemotherapy. • Oral care/rinses is one of the effective strategies to prevent or treat oral mucositis.

OBJECTIVE OF THE STUDY Comparing the effects of Pre and Post implementation of oral care kits on chemotherapy treated patients. • Groups: • Pre = July 2009 to June 2010 • Post = July 2010 to June 2011

INFORMATION ABOUT ORIGINAL DATA • Oral mucositis assessment • Total Score “ 0 -16” • “ 0”=Normal • Documented on admission and then at least twice a day. • Missing data exists.

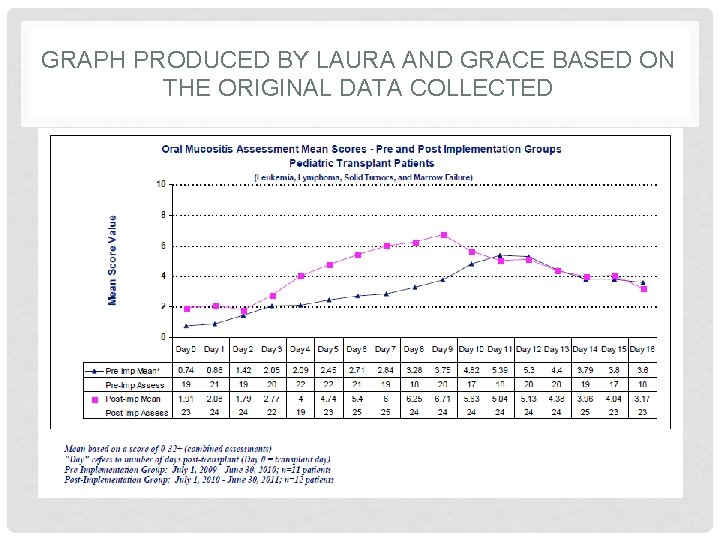
GRAPH PRODUCED BY LAURA AND GRACE BASED ON THE ORIGINAL DATA COLLECTED

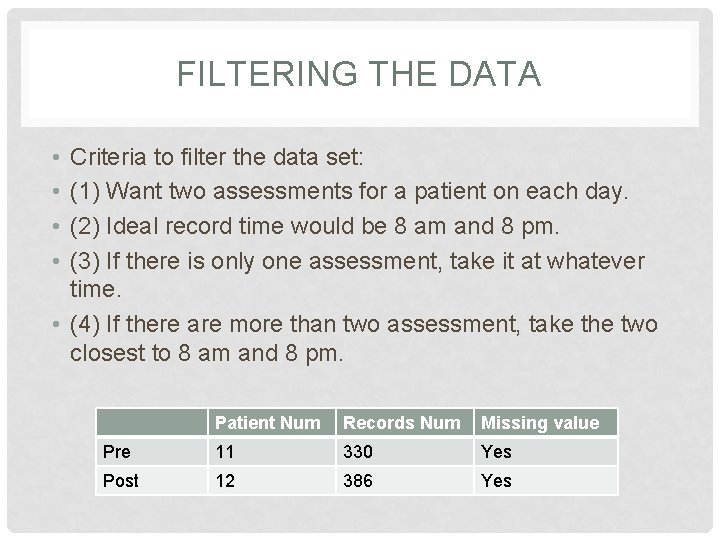
FILTERING THE DATA • • Criteria to filter the data set: (1) Want two assessments for a patient on each day. (2) Ideal record time would be 8 am and 8 pm. (3) If there is only one assessment, take it at whatever time. • (4) If there are more than two assessment, take the two closest to 8 am and 8 pm. Patient Num Records Num Missing value Pre 11 330 Yes Post 12 386 Yes

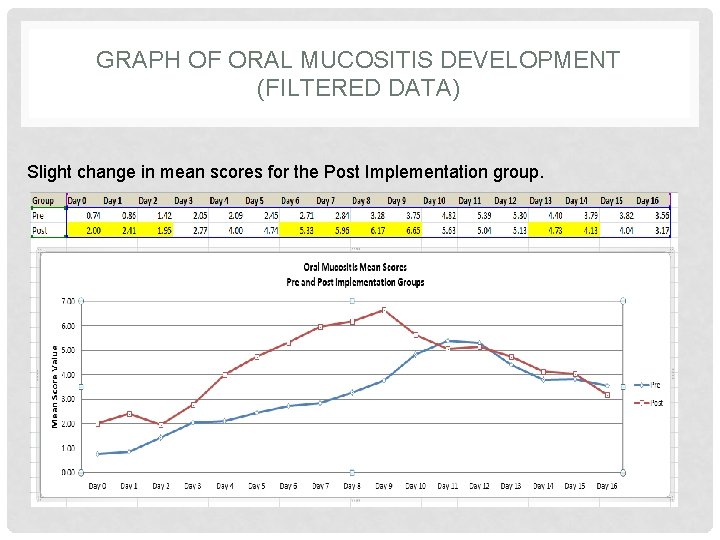
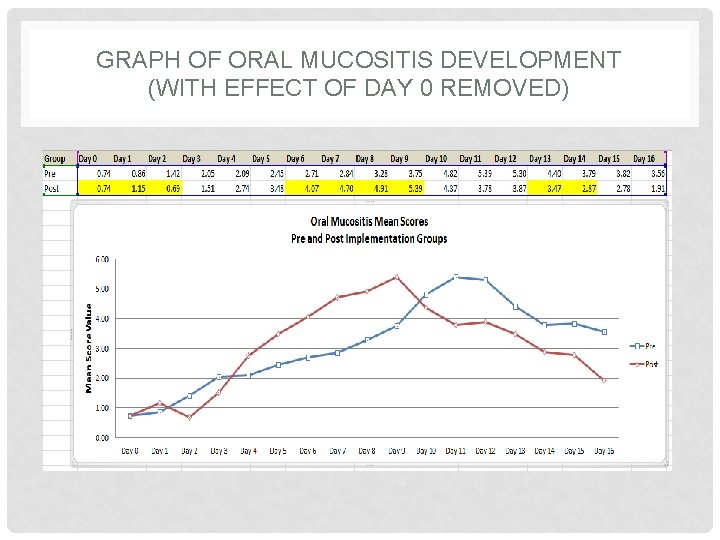
GRAPH OF ORAL MUCOSITIS DEVELOPMENT (FILTERED DATA) Slight change in mean scores for the Post Implementation group.

GRAPH OF ORAL MUCOSITIS DEVELOPMENT (WITH EFFECT OF DAY 0 REMOVED)

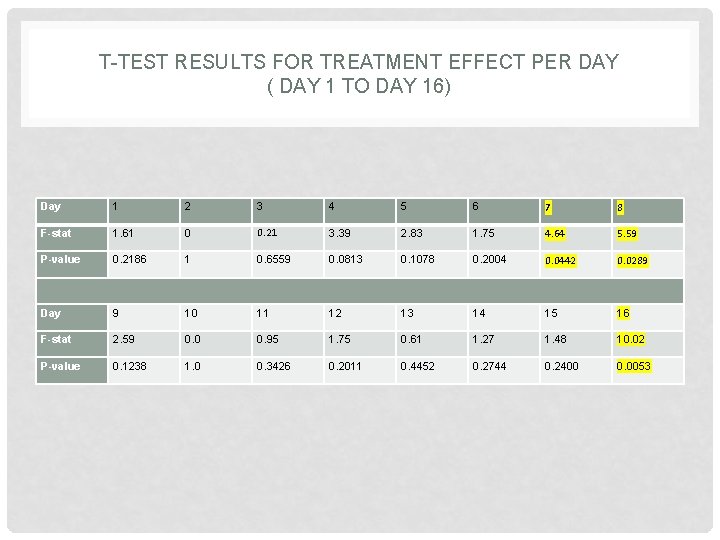
T-TEST RESULTS FOR TREATMENT EFFECT PER DAY ( DAY 1 TO DAY 16) Day 1 2 3 4 5 6 7 8 F-stat 1. 61 0 0. 21 3. 39 2. 83 1. 75 4. 64 5. 59 P-value 0. 2186 1 0. 6559 0. 0813 0. 1078 0. 2004 0. 0442 0. 0289 Day 9 10 11 12 13 14 15 16 F-stat 2. 59 0. 0 0. 95 1. 75 0. 61 1. 27 1. 48 10. 02 P-value 0. 1238 1. 0 0. 3426 0. 2011 0. 4452 0. 2744 0. 2400 0. 0053

T-TEST RESULTS FOR TREATMENT EFFECT PER DAY ( DAY 1 TO DAY 16) CONT’D • Except for days 7, 8, 16 all the t-tests are non significant at 0. 05. • For an appropriate analysis, we need to take into account multiple comparison and use bonferroni adjustment to conclude about the t-tests.

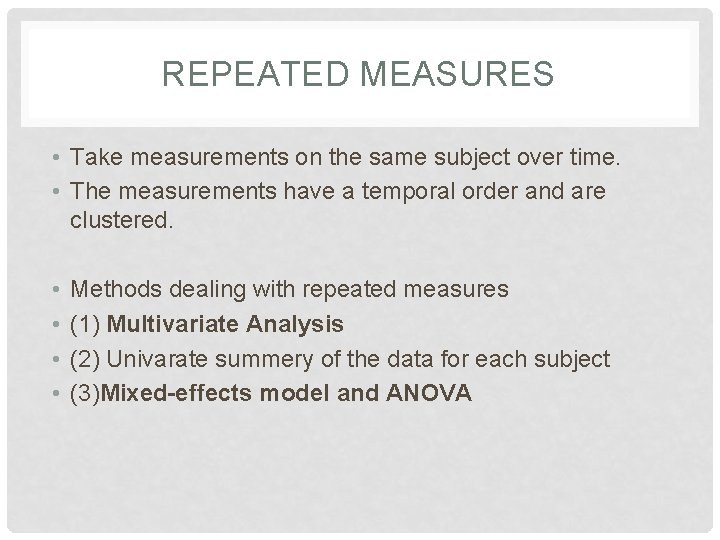
REPEATED MEASURES • Take measurements on the same subject over time. • The measurements have a temporal order and are clustered. • • Methods dealing with repeated measures (1) Multivariate Analysis (2) Univarate summery of the data for each subject (3)Mixed-effects model and ANOVA

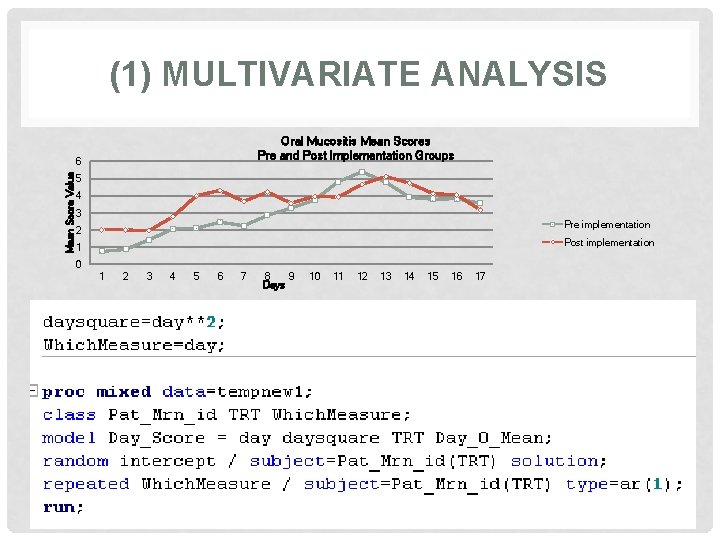
(1) MULTIVARIATE ANALYSIS Oral Mucositis Mean Scores Pre and Post Implementation Groups Mean Score Value 6 5 4 3 Pre implementation 2 Post implementation 1 0 1 2 3 4 5 6 7 8 9 Days 10 11 12 13 14 15 16 17

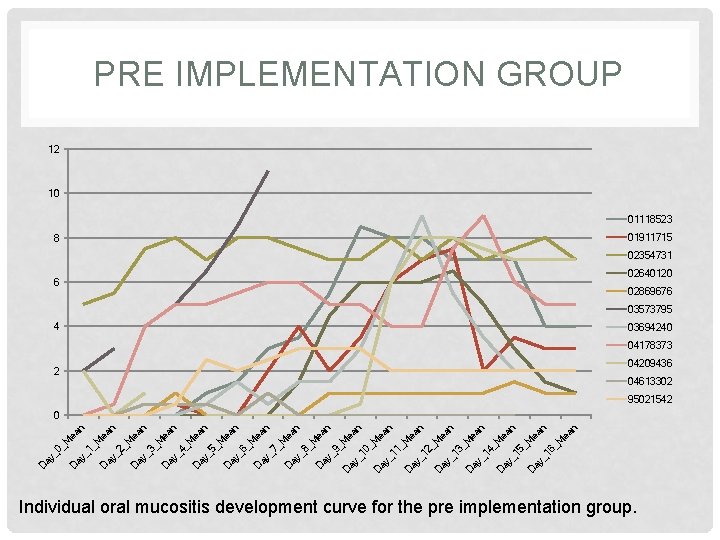
PRE IMPLEMENTATION GROUP 12 10 01118523 01911715 8 02354731 02640120 6 02869676 03573795 4 03694240 04178373 04209436 2 04613302 95021542 n _M ea n 16 ea ay _ D D ay _ 15 _M M ea n n 4_ _1 D ay 13 _M ea n D ay _ 12 ea n _M ea 11 _M D ay _ ea n 10 _M _9 D ay ay _ ea n _M D ea n ay _8 _M D ay D _6 ay D _7 _M ea n ay _5 _M D ea n ay _4 _M D ea n _3 ay _2 _M D ea n ay D _1 ay D D ay _0 _M _M ea n 0 Individual oral mucositis development curve for the pre implementation group.

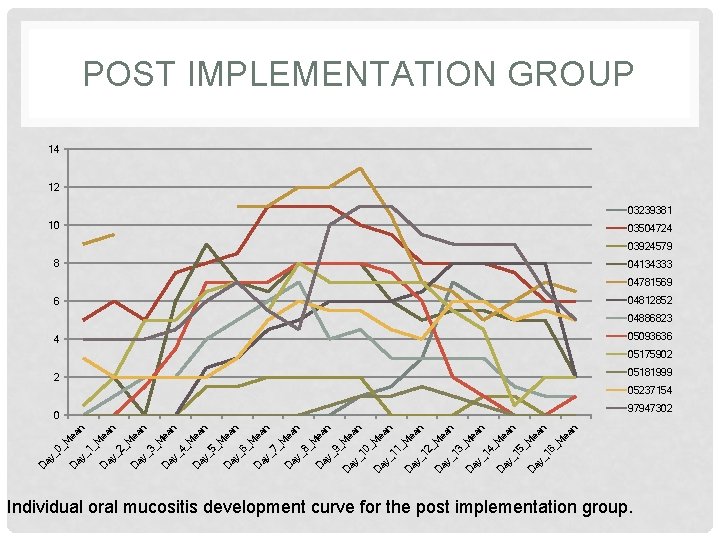
POST IMPLEMENTATION GROUP 14 12 03239381 10 03504724 03924579 8 04134333 04781569 04812852 6 04886823 05093636 4 05175902 05181999 2 05237154 97947302 n _M ea n 16 ea ay _ D D ay _ 15 _M M ea n n 4_ _1 D ay 13 _M ea n D ay _ 12 ea n _M ea 11 _M D ay _ ea n 10 _M _9 D ay ay _ ea n _M D ea n ay _8 _M D ay D _6 ay D _7 _M ea n ay _5 _M D ea n ay _4 _M D ea n _3 ay _2 _M D ea n ay D _1 ay D D ay _0 _M _M ea n 0 Individual oral mucositis development curve for the post implementation group.

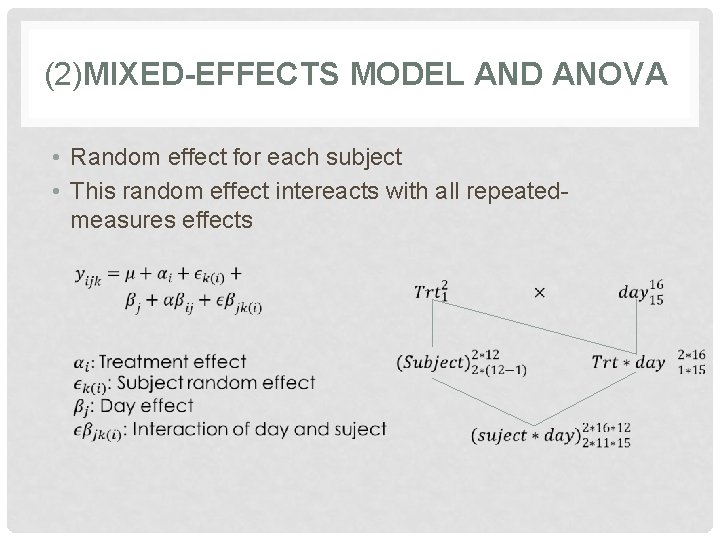
(2)MIXED-EFFECTS MODEL AND ANOVA • Random effect for each subject • This random effect intereacts with all repeatedmeasures effects

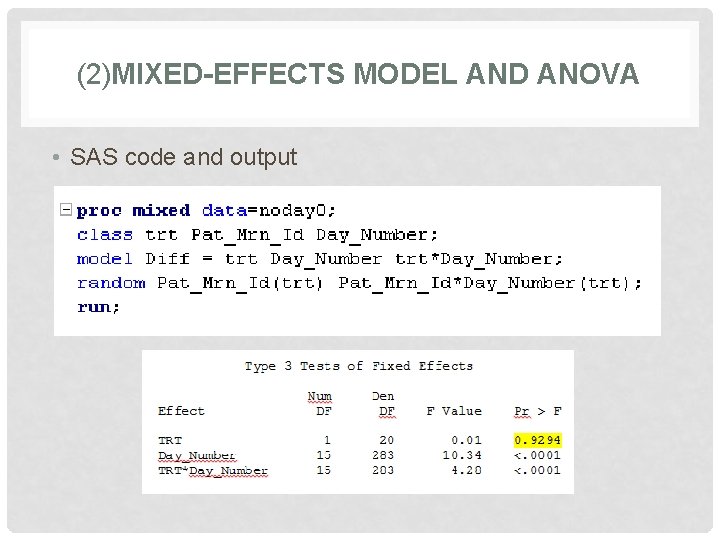
(2)MIXED-EFFECTS MODEL AND ANOVA • SAS code and output

CONCLUSIONS • There is no significant difference for the Pre and Post treatments in preventing oral mucositis.
 Infant oral health care
Infant oral health care Infant oral health care
Infant oral health care Health care levels primary secondary tertiary
Health care levels primary secondary tertiary Health and social care values unit 2
Health and social care values unit 2 Asante test kits
Asante test kits Kits nfi
Kits nfi 1990 aasta loom
1990 aasta loom Qrp club kits
Qrp club kits Kits oreillettes arbitrage pro
Kits oreillettes arbitrage pro Marilyn burns fraction kit
Marilyn burns fraction kit Washington homeopathic kits
Washington homeopathic kits Seismic aircraft cable suppliers
Seismic aircraft cable suppliers Dicks sporting goods first aid kits
Dicks sporting goods first aid kits First robotics suppliers
First robotics suppliers Biozek corona rapid test
Biozek corona rapid test Kits courseweb
Kits courseweb Naco kit 6 yellow
Naco kit 6 yellow Medicare health outcomes survey
Medicare health outcomes survey