HIV1 Rapid Test for Recent Infection Asante Rapid

















- Slides: 43

HIV-1 Rapid Test for Recent Infection: Asante Rapid Test for Recent Infection (RTRI) SAMPLE COLLECTION AND STEP-WISE PROCEDURE FOR ASANTE RTRI International Laboratory Branch Division of Global HIV/AIDS & Tuberculosis Centers for Disease Control and Prevention

2 Outline • Describe the procedure for collection of whole blood specimens • Venous • Finger prick • Dried blood spots (DBS, for VL testing only) • Explain the specimen handling and storage requirements • Understand the stepwise procedure for Asante testing and interpretation of results

SPECIMEN COLLECTION: WHOLE BLOOD

4 Different Procedures for Whole Blood Collection Venous Blood Finger Prick

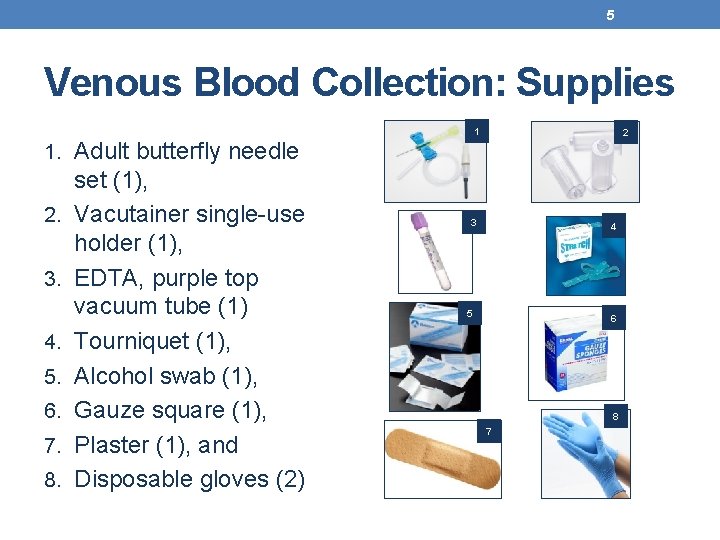
5 Venous Blood Collection: Supplies 1 2 1. Adult butterfly needle 2. 3. 4. 5. 6. 7. 8. set (1), Vacutainer single-use holder (1), EDTA, purple top vacuum tube (1) Tourniquet (1), Alcohol swab (1), Gauze square (1), Plaster (1), and Disposable gloves (2) 3 4 5 6 8 7

6 Other Supplies Needed: Venous Blood Collection • Sharps container (1) • Specimen label (2) • A pre-printed barcode label to affix to the specimen tube OR • An ultra-fine permanent marker to document the participant’s unique ID on the specimen tube 1 2 a 2 b

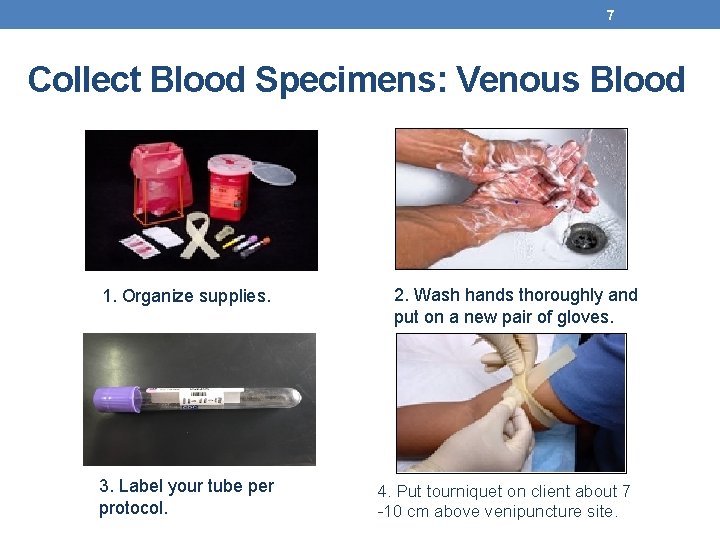
7 Collect Blood Specimens: Venous Blood 1. Organize supplies. 3. Label your tube per protocol. 2. Wash hands thoroughly and put on a new pair of gloves. 4. Put tourniquet on client about 7 -10 cm above venipuncture site.

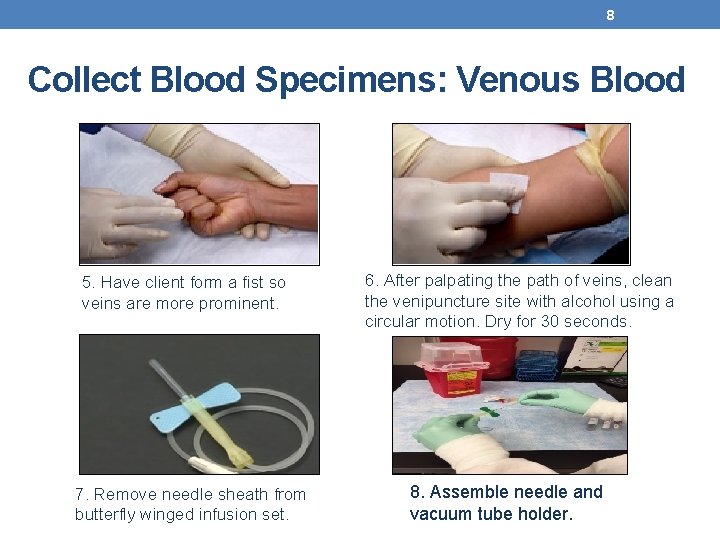
8 Collect Blood Specimens: Venous Blood 5. Have client form a fist so veins are more prominent. 7. Remove needle sheath from butterfly winged infusion set. 6. After palpating the path of veins, clean the venipuncture site with alcohol using a circular motion. Dry for 30 seconds. 8. Assemble needle and vacuum tube holder.

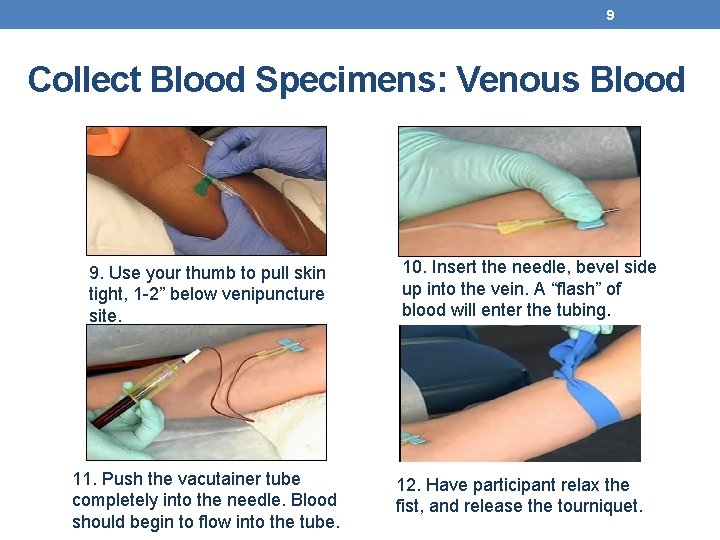
9 Collect Blood Specimens: Venous Blood 9. Use your thumb to pull skin tight, 1 -2” below venipuncture site. 11. Push the vacutainer tube completely into the needle. Blood should begin to flow into the tube. 10. Insert the needle, bevel side up into the vein. A “flash” of blood will enter the tubing. 12. Have participant relax the fist, and release the tourniquet.

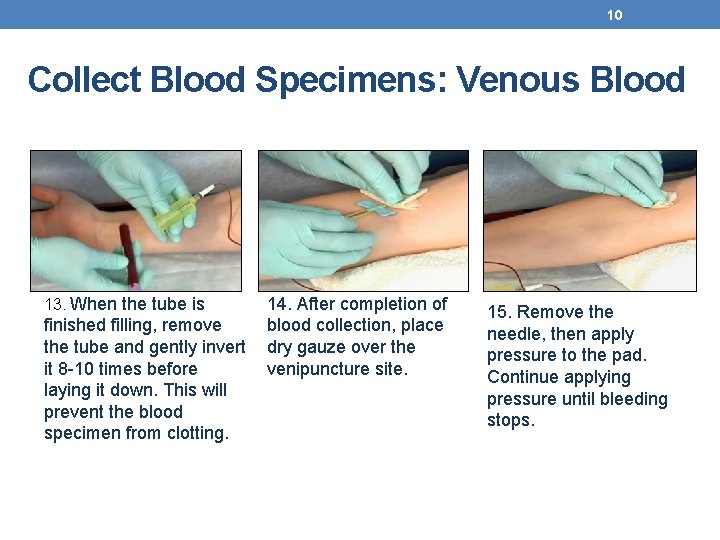
10 Collect Blood Specimens: Venous Blood 13. When the tube is finished filling, remove the tube and gently invert it 8 -10 times before laying it down. This will prevent the blood specimen from clotting. 14. After completion of blood collection, place dry gauze over the venipuncture site. 15. Remove the needle, then apply pressure to the pad. Continue applying pressure until bleeding stops.

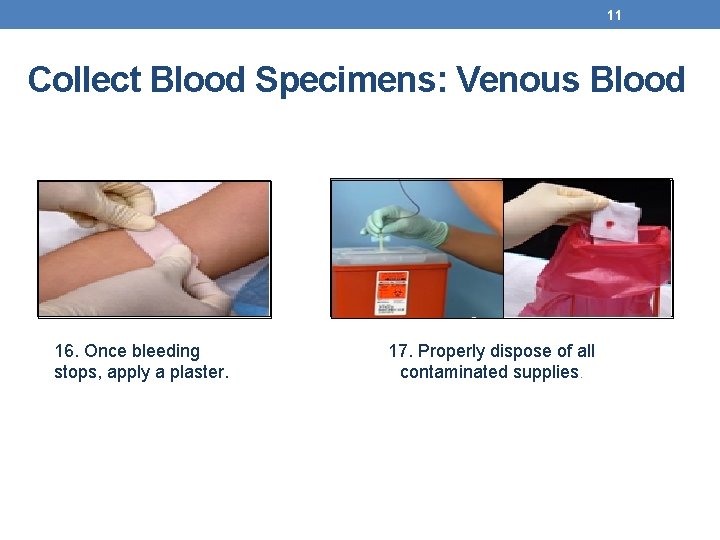
11 Collect Blood Specimens: Venous Blood 16. Once bleeding stops, apply a plaster. 17. Properly dispose of all contaminated supplies.

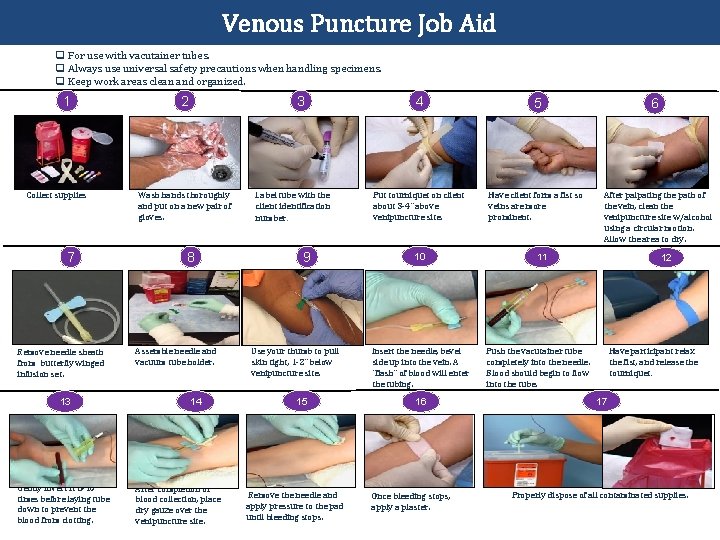
Venous Puncture Job Aid q For use with vacutainer tubes. q Always use universal safety precautions when handling specimens. q Keep work areas clean and organized. 1 Collect supplies 7 Remove needle sheath from butterfly winged infusion set. 13 Gently invert it 8 -10 times before laying tube down to prevent the blood from clotting. 2 3 Wash hands thoroughly and put on a new pair of gloves. 8 Assemble needle and vacuum tube holder. 14 After completion of blood collection, place dry gauze over the venipuncture site. Label tube with the client identification number. 9 Use your thumb to pull skin tight, 1 -2” below venipuncture site. 15 Remove the needle and apply pressure to the pad until bleeding stops. 4 5 Put tourniquet on client about 3 -4” above venipuncture site. Have client form a fist so veins are more prominent. 10 Insert the needle, bevel side up into the vein. A “flash” of blood will enter the tubing. 16 Once bleeding stops, apply a plaster. 6 After palpating the path of the vein, clean the venipuncture site w/alcohol using a circular motion. Allow the area to dry. 11 12 Push the vacutainer tube completely into the needle. Blood should begin to flow into the tube. Have participant relax the fist, and release the tourniquet. 17 Properly dispose of all contaminated supplies.

13 Blood Collection Supplies: Finger Prick 1 2 1. Lancet(1), 2. Alcohol swab (1), 3. Gauze square (1), 3 4 4. Loop (1) (provided by kit), 5. Gloves (2), 6. Optional: Plaster (1) 5 6

14 Other Supplies Needed: Finger Prick • Sharps container (1) 1 • Biohazard waste container • An ultra-fine permanent marker to document the participant’s unique ID on the specimen tube 2 3

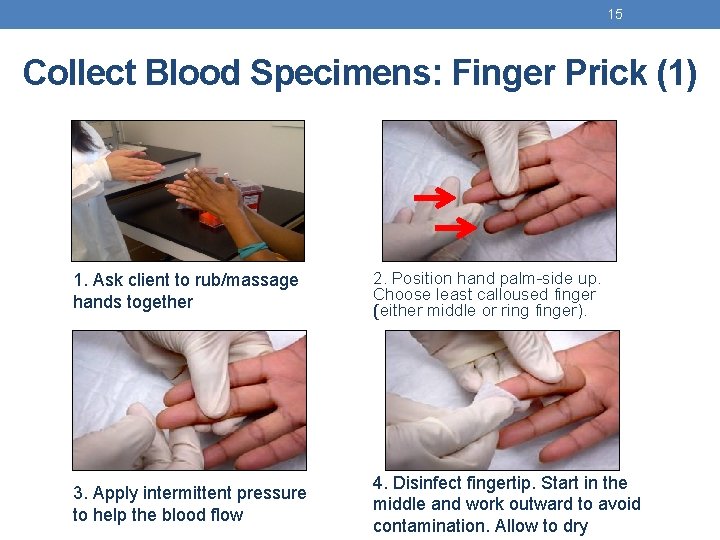
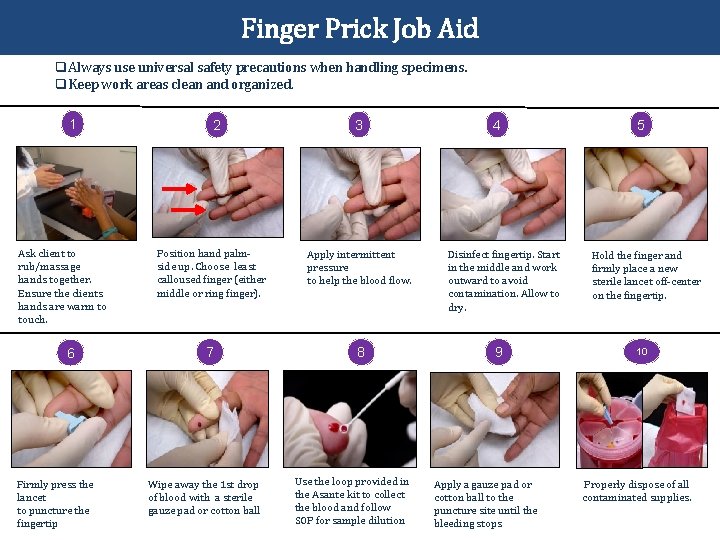
15 Collect Blood Specimens: Finger Prick (1) 1. Ask client to rub/massage hands together 2. Position hand palm-side up. Choose least calloused finger (either middle or ring finger). 3. Apply intermittent pressure to help the blood flow 4. Disinfect fingertip. Start in the middle and work outward to avoid contamination. Allow to dry

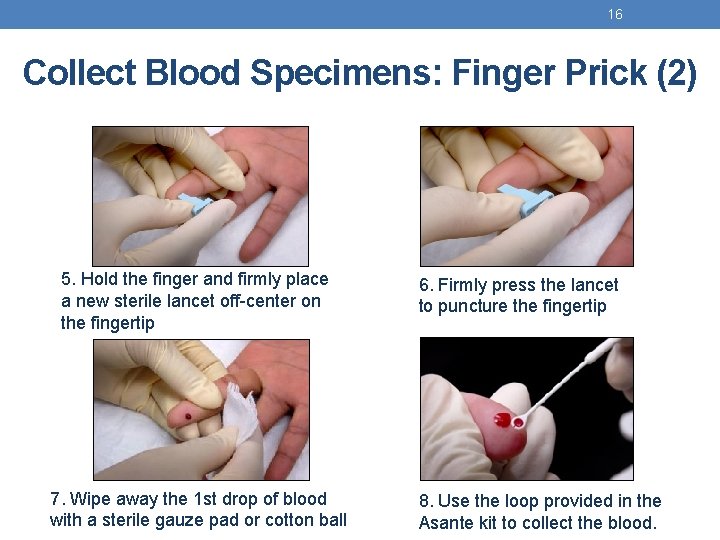
16 Collect Blood Specimens: Finger Prick (2) 5. Hold the finger and firmly place a new sterile lancet off-center on the fingertip 7. Wipe away the 1 st drop of blood with a sterile gauze pad or cotton ball 6. Firmly press the lancet to puncture the fingertip 8. Use the loop provided in the Asante kit to collect the blood.

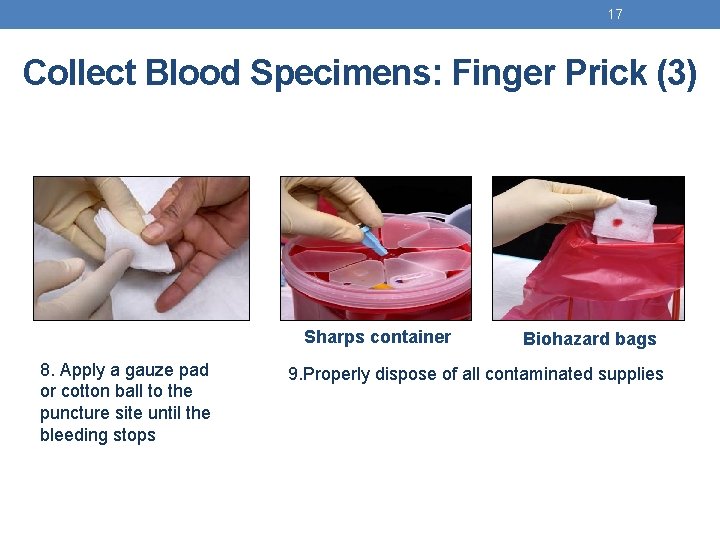
17 Collect Blood Specimens: Finger Prick (3) Sharps container 8. Apply a gauze pad or cotton ball to the puncture site until the bleeding stops Biohazard bags 9. Properly dispose of all contaminated supplies

Finger Prick Job Aid q Always use universal safety precautions when handling specimens. q Keep work areas clean and organized. 1 Ask client to rub/massage hands together. Ensure the clients hands are warm to touch. 6 Firmly press the lancet to puncture the fingertip 2 3 Position hand palmside up. Choose least calloused finger (either middle or ring finger). Apply intermittent pressure to help the blood flow. 7 8 Wipe away the 1 st drop of blood with a sterile gauze pad or cotton ball Use the loop provided in the Asante kit to collect the blood and follow SOP for sample dilution 4 Disinfect fingertip. Start in the middle and work outward to avoid contamination. Allow to dry. 9 Apply a gauze pad or cotton ball to the puncture site until the bleeding stops 5 Hold the finger and firmly place a new sterile lancet off-center on the fingertip. 10 Properly dispose of all contaminated supplies.

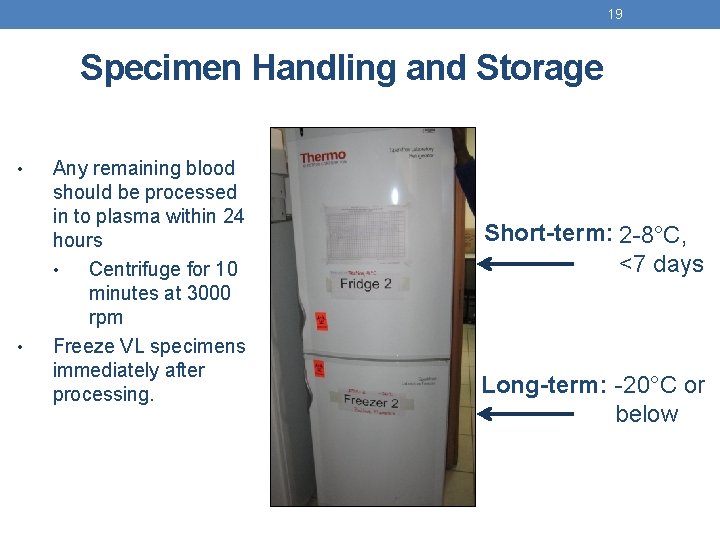
19 Specimen Handling and Storage • • Any remaining blood should be processed in to plasma within 24 hours • Centrifuge for 10 minutes at 3000 rpm Freeze VL specimens immediately after processing. Short-term: 2 -8°C, <7 days Long-term: -20°C or below

20 Countries with Capacity for Viral Load Testing • Specialized testing, such as viral load testing, may be conducted on client specimens. • Follow country-specific guidelines for specimen collection, processing, storage, and transport for VL testing. • In some countries, the specimen of choice is DBS. • Prepare 1 DBS card per sample using Whatman 903 filter paper. • Load 75 µl of blood per circle. Two complete circles are better than 5 incomplete circles.

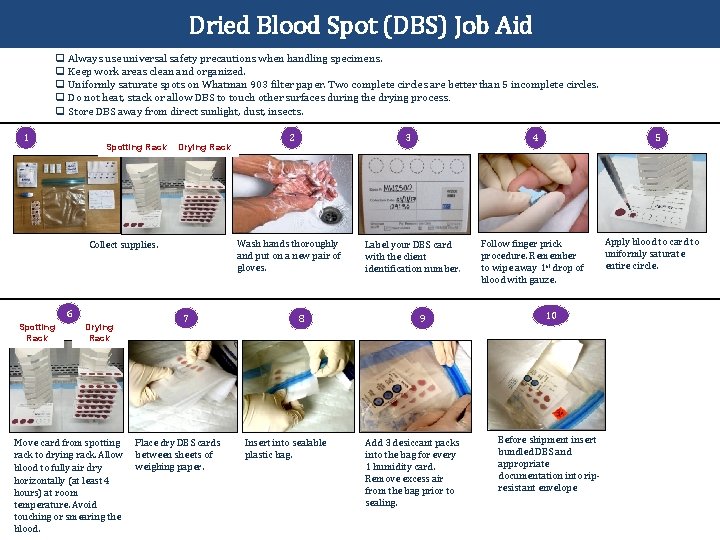
Dried Blood Spot (DBS) Job Aid q Always use universal safety precautions when handling specimens. q Keep work areas clean and organized. q Uniformly saturate spots on Whatman 903 filter paper. Two complete circles are better than 5 incomplete circles. q Do not heat, stack or allow DBS to touch other surfaces during the drying process. q Store DBS away from direct sunlight, dust, insects. 1 Spotting Rack Drying Rack 6 Drying Rack 3 Wash hands thoroughly and put on a new pair of gloves. Collect supplies. Spotting Rack 2 7 Move card from spotting Place dry DBS cards rack to drying rack. Allow between sheets of weighing paper. blood to fully air dry horizontally (at least 4 hours) at room temperature. Avoid touching or smearing the blood. 8 Insert into sealable plastic bag. 4 Label your DBS card with the client identification number. 9 Add 3 desiccant packs into the bag for every 1 humidity card. Remove excess air from the bag prior to sealing. 5 Follow finger prick procedure. Remember to wipe away 1 st drop of blood with gauze. 10 Before shipment insert bundled DBS and appropriate documentation into ripresistant envelope Apply blood to card to uniformly saturate entire circle.

STEP-BY-STEP PROCEDURE FOR ASANTE RTRI

23 Before You Start Testing… • Check kit before use, use only kits that have not expired and test devices that are not damaged. • Keep kits stored at 2 - 30°C • If kits were stored below room temperature, bring them to room temperature before testing • Bring stored specimens from refrigerator to room temperature prior to use. • Always use universal safety precautions when handling specimens. • Keep work areas clean and organized.

1 Assemble Test Kit and Supplies 24

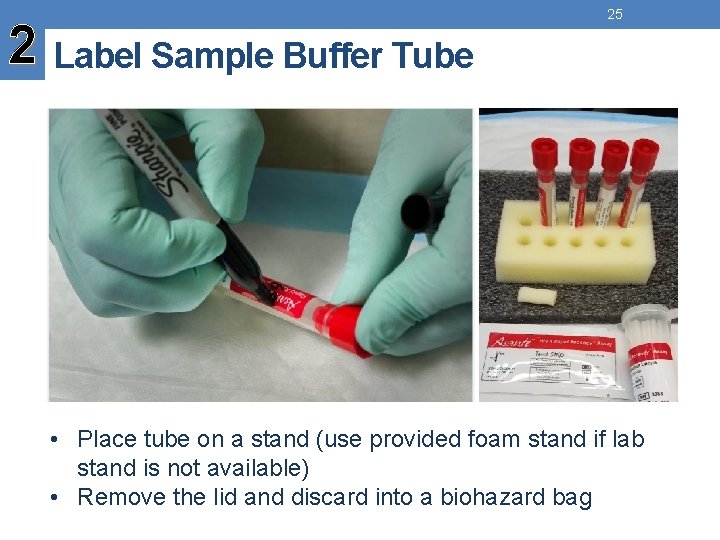
2 Label Sample Buffer Tube 25 • Place tube on a stand (use provided foam stand if lab stand is not available) • Remove the lid and discard into a biohazard bag

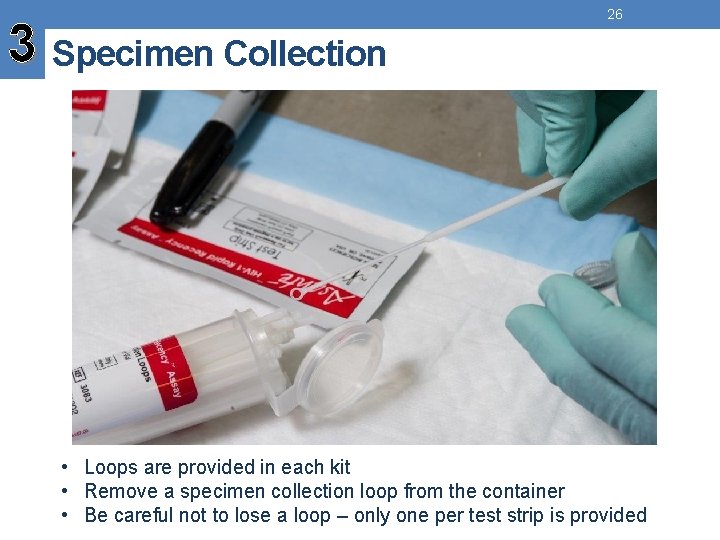
3 Specimen Collection 26 • Loops are provided in each kit • Remove a specimen collection loop from the container • Be careful not to lose a loop – only one per test strip is provided

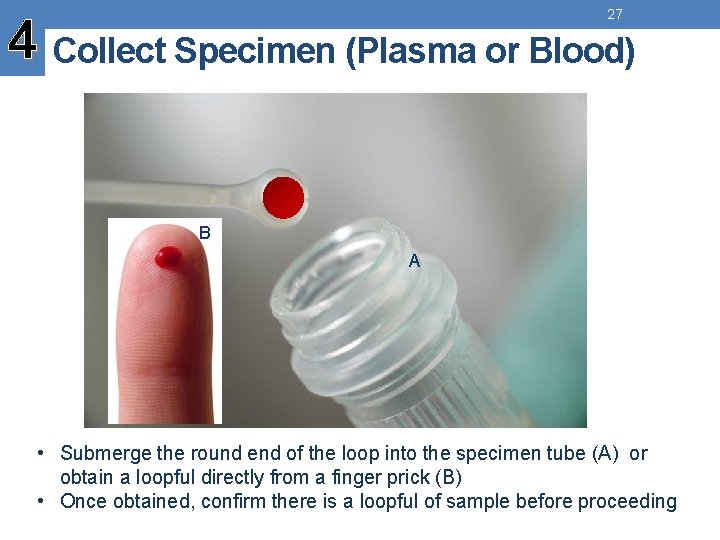
27 4 Collect Specimen (Plasma or Blood) B A • Submerge the round end of the loop into the specimen tube (A) or obtain a loopful directly from a finger prick (B) • Once obtained, confirm there is a loopful of sample before proceeding

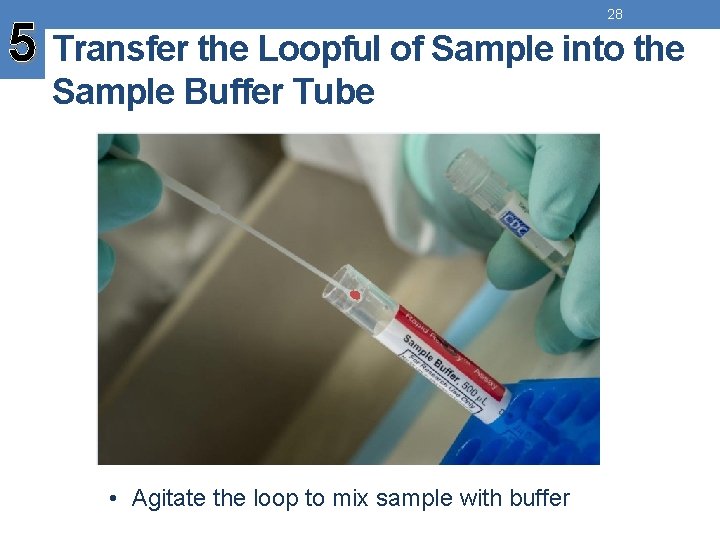
28 5 Transfer the Loopful of Sample into the Sample Buffer Tube • Agitate the loop to mix sample with buffer

29 6 Discard the Specimen Collection Loop into a Biohazard Bag.

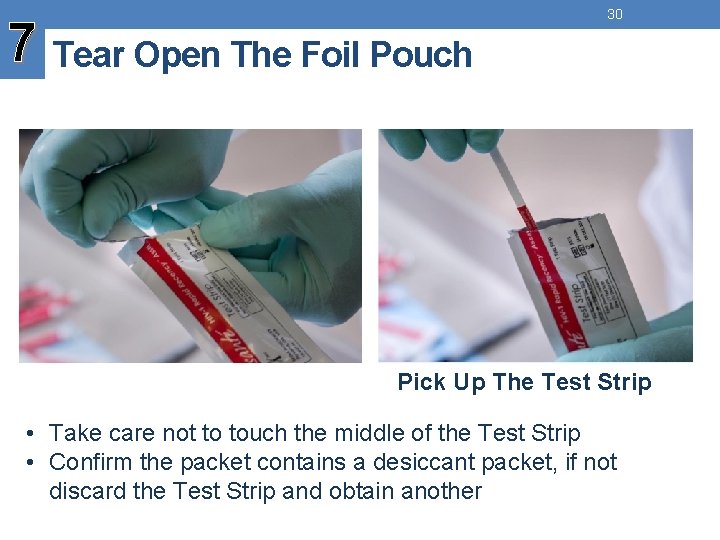
7 Tear Open The Foil Pouch 30 Pick Up The Test Strip • Take care not to touch the middle of the Test Strip • Confirm the packet contains a desiccant packet, if not discard the Test Strip and obtain another

31 8 Insert the Test Strip into the Liquid in the Sample Buffer Tube • Arrows printed on the strip should be pointing toward the liquid.

9 Start Timer (20 Minutes) 32

33 10 Let the Sample Buffer with Test Strips Stand on the Rack

11 After Incubating for 20 Minutes 34 • Remove strip from the sample buffer tube • Touch the lower end of the strip to paper towel to drain any excess buffer • Lay it flat on the bench and read the results immediately • Record results and discard strip

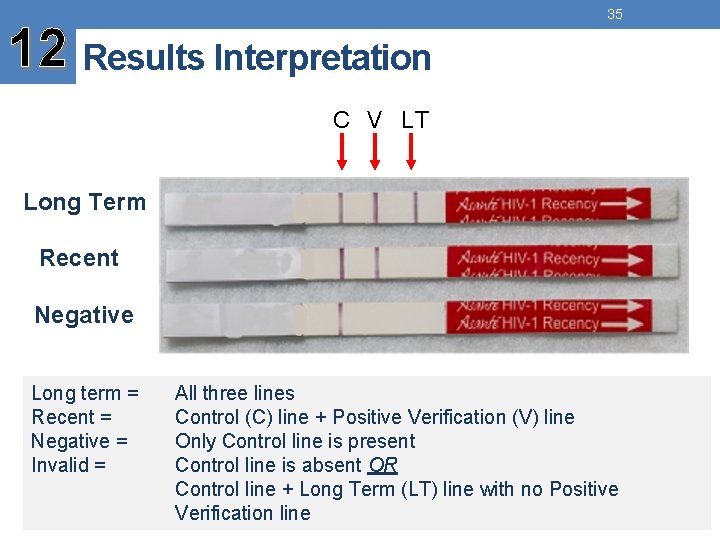
12 Results Interpretation 35 C V LT Long Term Recent Negative Long term = Recent = Negative = Invalid = All three lines Control (C) line + Positive Verification (V) line Only Control line is present Control line is absent OR Control line + Long Term (LT) line with no Positive Verification line

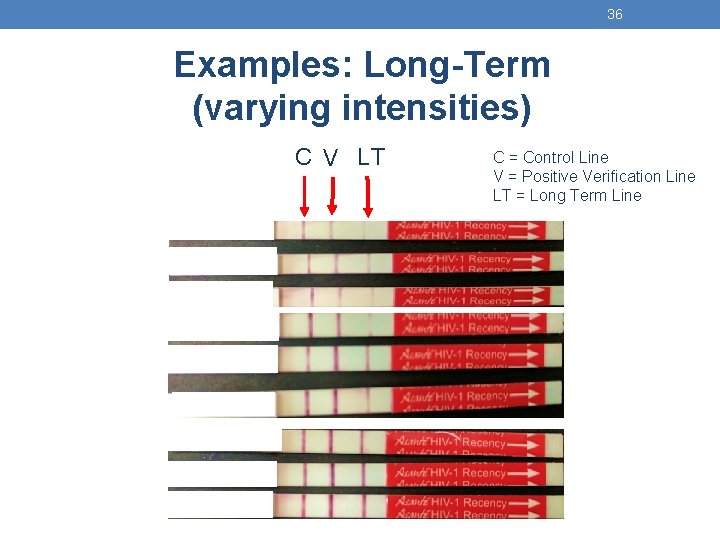
36 Examples: Long-Term (varying intensities) C V LT C = Control Line V = Positive Verification Line LT = Long Term Line

37 Examples: Recent (varying intensities) C V LT C = Control Line V = Positive Verification Line LT = Long Term Line

38 Examples: Invalid Results C V LT C = Control Line V = Positive Verification Line LT = Long Term Line Note that this is a rare event, but should always be recorded

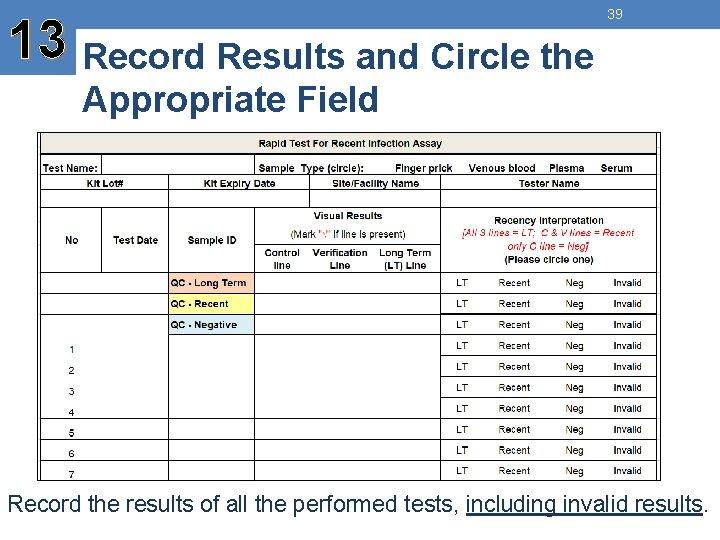
13 Record Results and Circle the 39 Appropriate Field Record the results of all the performed tests, including invalid results.

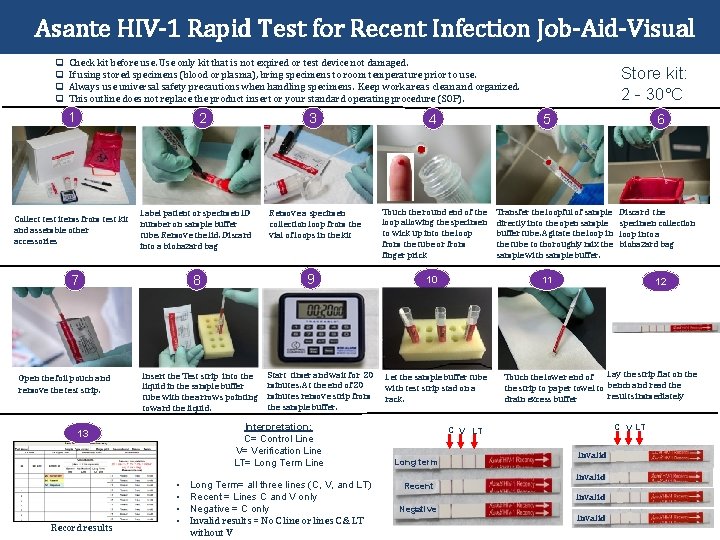
Asante HIV-1 Rapid Test for Recent Infection Job-Aid-Visual q q Check kit before use. Use only kit that is not expired or test device not damaged. If using stored specimens (blood or plasma), bring specimens to room temperature prior to use. Always use universal safety precautions when handling specimens. Keep work areas clean and organized. This outline does not replace the product insert or your standard operating procedure (SOP). 1 2 Collect test items from test kit and assemble other accessories Label patient or specimen ID number on sample buffer tube. Remove the lid. Discard into a biohazard bag Open the foil pouch and remove the test strip. Insert the Test strip into the liquid in the sample buffer tube with the arrows pointing toward the liquid. Remove a specimen collection loop from the vial of loops in the kit Touch the round end of the loop allowing the specimen to wick up into the loop from the tube or from finger prick Start timer and wait for 20 minutes. At the end of 20 minutes remove strip from the sample buffer. Interpretation: C= Control Line V= Verification Line LT= Long Term Line 13 Record results 4 9 8 7 3 • • Long Term= all three lines (C, V, and LT) Recent = Lines C and V only Negative = C only Invalid results = No C line or lines C & LT without V Store kit: 2 - 30°C 5 10 6 Transfer the loopful of sample directly into the open sample buffer tube. Agitate the loop in the tube to thoroughly mix the sample with sample buffer. 11 Let the sample buffer tube with test strip stad on a rack. 12 Touch the lower end of Lay the strip flat on the strip to paper towel to bench and read the results immediately drain excess buffer C V LT Long term Recent Invalid Negative Discard the specimen collection loop into a biohazard bag Invalid

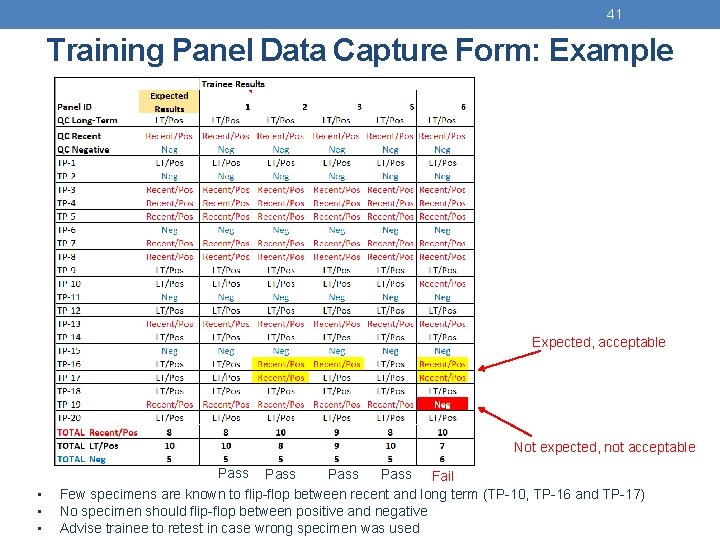
41 Training Panel Data Capture Form: Example Expected, acceptable Not expected, not acceptable Pass • • • Pass Fail Pass Few specimens are known to flip-flop between recent and long term (TP-10, TP-16 and TP-17) No specimen should flip-flop between positive and negative Advise trainee to retest in case wrong specimen was used

42 Review • What methods are used to collect whole blood? • What are the techniques used to collect whole blood from a finger prick ? • At what temperature, and for how long, should specimens be stored? • When would DBS specimens be collected? • What are the steps when performing the Asante recency rapid test?

Thank You!