Oneyear outcomes in patients taking aspirin vs P



- Slides: 12

One-year outcomes in patients taking aspirin vs. P 2 Y 12 monotherapy after PCI: Analysis from the Onyx ONE trial Azeem Latib, Stephan Windecker, Elvin Kedhi, David Kandzari, Ajay Kirtane, Roxana Mehran, Matthew Price, Sandeep Brar, Lisa Bousquette, Te-Hsin Lung, Alexandre Abizaid, Daniel Simon, Steve Worthley, Azfar Zaman, Gregg W. Stone

Potential conflicts of interest Speaker's name : Azeem Latib ☑ I have the following potential conflicts of interest to declare: Receipt of honoraria or consultation fees: Abbott, Edwards, Medtronic, Philips Azeem Latib

Why this study? • The Onyx ONE trial demonstrated that Resolute Onyx™ is safe and effective in complex high bleeding risk patients who receive 1 -month DAPT 1 • The current analysis examined if there are differences in outcomes at 1 -year with prescribed SAPT monotherapy (aspirin vs. oral P 2 Y 12 inhibitor (P 2 Y 12 i)) after discontinuation of DAPT at 1 -month Azeem Latib ONYX ONE SAPT analysis 1 Windecker S, et al. N Engl J Med. 2020; 382(13): 1208 -1218.

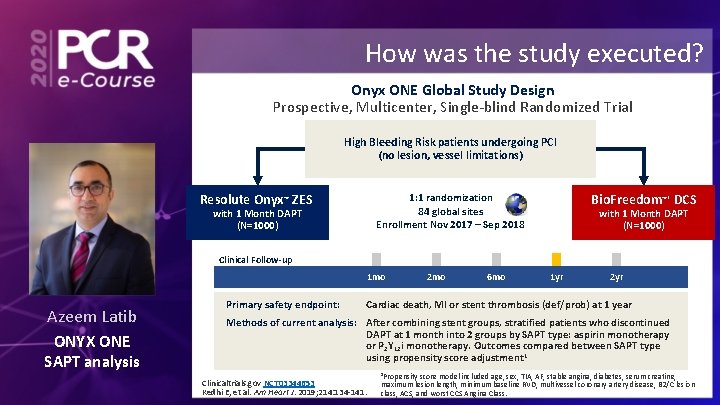
How was the study executed? Onyx ONE Global Study Design Prospective, Multicenter, Single-blind Randomized Trial High Bleeding Risk patients undergoing PCI (no lesion, vessel limitations) 1: 1 randomization 84 global sites Enrollment Nov 2017 – Sep 2018 Resolute Onyx™ ZES with 1 Month DAPT (N=1000) Bio. Freedom™* DCS with 1 Month DAPT (N=1000) Clinical Follow-up 1 mo Azeem Latib ONYX ONE SAPT analysis Primary safety endpoint: 2 mo 6 mo 1 yr 2 yr Cardiac death, MI or stent thrombosis (def/prob) at 1 year Methods of current analysis: After combining stent groups, stratified patients who discontinued DAPT at 1 month into 2 groups by SAPT type: aspirin monotherapy or P 2 Y 12 i monotherapy. Outcomes compared between SAPT type using propensity score adjustment 1 Clinicaltrials. gov NCT 03344653 Kedhi E, et al. Am Heart J. 2019; 214: 134 -141. 1 Propensity score model included age, sex, TIA, AF, stable angina, diabetes, serum creatine, maximum lesion length, minimum baseline RVD, multivessel coronary artery disease, B 2/C lesion class, ACS, and worst CCS Angina Class.

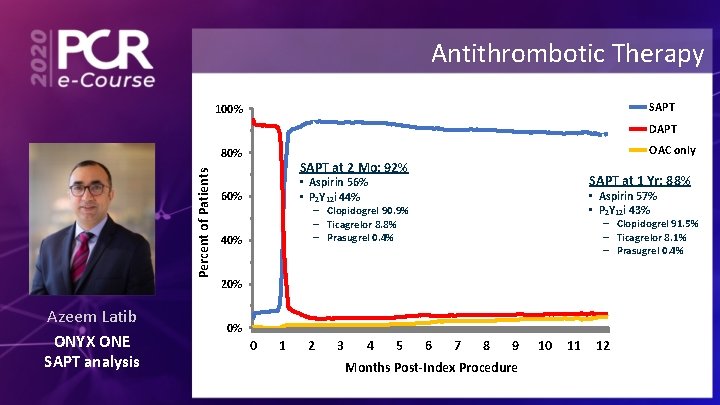
Antithrombotic Therapy SAPT 100% DAPT OAC only Percent of Patients 80% Azeem Latib ONYX ONE SAPT analysis SAPT at 2 Mo: 92% SAPT at 1 Yr: 88% • Aspirin 56% • P 2 Y 12 i 44% 60% • Aspirin 57% • P 2 Y 12 i 43% – Clopidogrel 90. 9% – Ticagrelor 8. 8% – Prasugrel 0. 4% 40% – Clopidogrel 91. 5% – Ticagrelor 8. 1% – Prasugrel 0. 4% 20% 0% 0 1 2 3 4 5 6 7 8 9 Months Post-Index Procedure 10 11 12

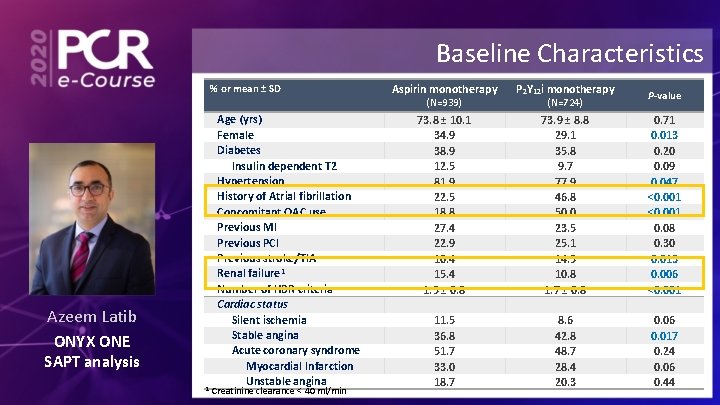
Baseline Characteristics % or mean ± SD Aspirin monotherapy P 2 Y 12 i monotherapy 73. 8 ± 10. 1 34. 9 38. 9 12. 5 81. 9 22. 5 18. 8 27. 4 22. 9 10. 4 15. 4 1. 5 ± 0. 8 73. 9 ± 8. 8 29. 1 35. 8 9. 7 77. 9 46. 8 50. 0 23. 5 25. 1 14. 5 10. 8 1. 7 ± 0. 8 0. 71 0. 013 0. 20 0. 09 0. 047 <0. 001 0. 08 0. 30 0. 013 0. 006 <0. 001 11. 5 36. 8 51. 7 33. 0 18. 7 8. 6 42. 8 48. 7 28. 4 20. 3 0. 06 0. 017 0. 24 0. 06 0. 44 (N=939) Azeem Latib ONYX ONE SAPT analysis 1 Age (yrs) Female Diabetes Insulin dependent T 2 Hypertension History of Atrial fibrillation Concomitant OAC use Previous MI Previous PCI Previous stroke/TIA Renal failure 1 Number of HBR criteria Cardiac status Silent ischemia Stable angina Acute coronary syndrome Myocardial Infarction Unstable angina Creatinine clearance < 40 ml/min (N=724) P-value

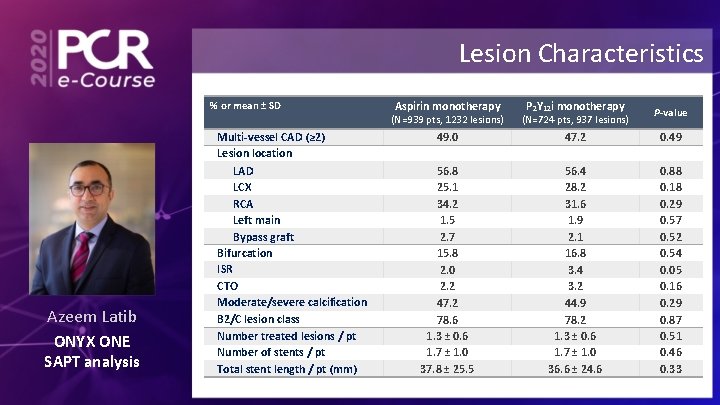
Lesion Characteristics % or mean ± SD Azeem Latib ONYX ONE SAPT analysis Multi-vessel CAD (≥ 2) Lesion location LAD LCX RCA Left main Bypass graft Bifurcation ISR CTO Moderate/severe calcification B 2/C lesion class Number treated lesions / pt Number of stents / pt Total stent length / pt (mm) Aspirin monotherapy P 2 Y 12 i monotherapy P-value (N=939 pts, 1232 lesions) (N=724 pts, 937 lesions) 49. 0 47. 2 0. 49 56. 8 25. 1 34. 2 1. 5 2. 7 15. 8 2. 0 2. 2 47. 2 78. 6 1. 3 ± 0. 6 1. 7 ± 1. 0 37. 8 ± 25. 5 56. 4 28. 2 31. 6 1. 9 2. 1 16. 8 3. 4 3. 2 44. 9 78. 2 1. 3 ± 0. 6 1. 7 ± 1. 0 36. 6 ± 24. 6 0. 88 0. 18 0. 29 0. 57 0. 52 0. 54 0. 05 0. 16 0. 29 0. 87 0. 51 0. 46 0. 33

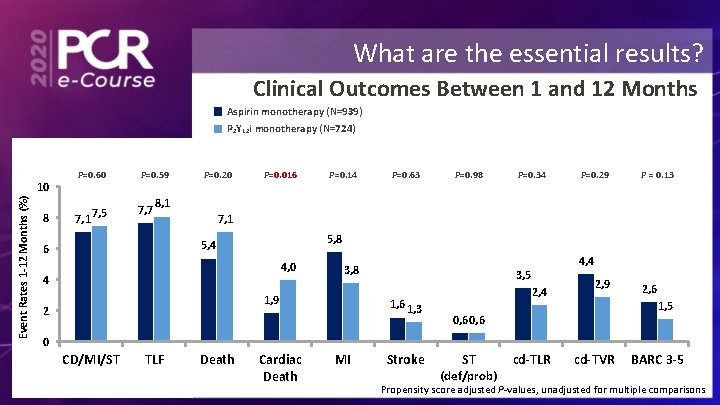
What are the essential results? Clinical Outcomes Between 1 and 12 Months Aspirin monotherapy (N=939) P 2 Y 12 i monotherapy (N=724) Event Rates 1 -12 Months (%) 10 8 P=0. 60 7, 1 7, 5 P=0. 59 7, 7 P=0. 20 P=0. 016 P=0. 14 P=0. 63 P=0. 98 P=0. 34 P=0. 29 P = 0. 13 8, 1 7, 1 5, 8 5, 4 6 4, 0 4 3, 8 1, 9 2 4, 4 3, 5 1, 6 1, 3 2, 4 2, 9 2, 6 1, 5 0, 6 0 CD/MI/ST TLF Death Cardiac Death MI Stroke ST (def/prob) cd-TLR cd-TVR BARC 3 -5 Propensity score adjusted P-values, unadjusted for multiple comparisons

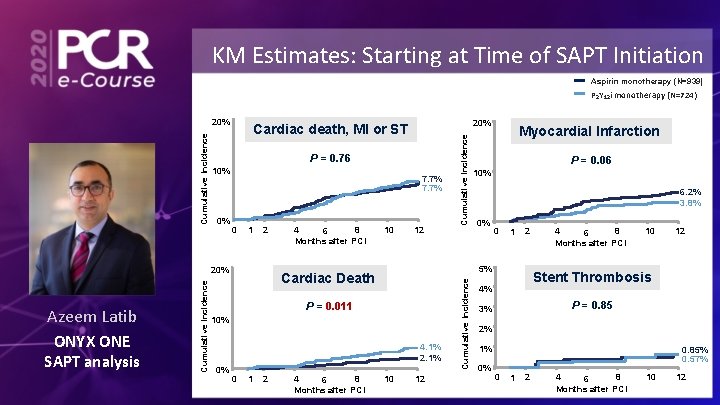
KM Estimates: Starting at Time of SAPT Initiation Aspirin monotherapy (N=939) P 2 Y 12 i monotherapy (N=724) 0% 7. 7% 0 1 2 Cumulative Incidence ONYX ONE SAPT analysis 4 8 6 Months after PCI 10 12 P = 0. 011 4. 1% 2. 1% 0 1 2 4 8 6 Months after PCI Myocardial Infarction P = 0. 06 10% 6. 2% 3. 8% 0% 0 1 2 5% Cardiac Death 10% 0% Cumulative Incidence P = 0. 76 10% 20% Azeem Latib 20% Cardiac death, MI or ST 10 12 Cumulative Incidence 20% 4 8 6 Months after PCI 10 12 Stent Thrombosis 4% P = 0. 85 3% 2% 1% 0% 0. 85% 0. 57% 0 1 2 4 8 6 Months after PCI 10 12

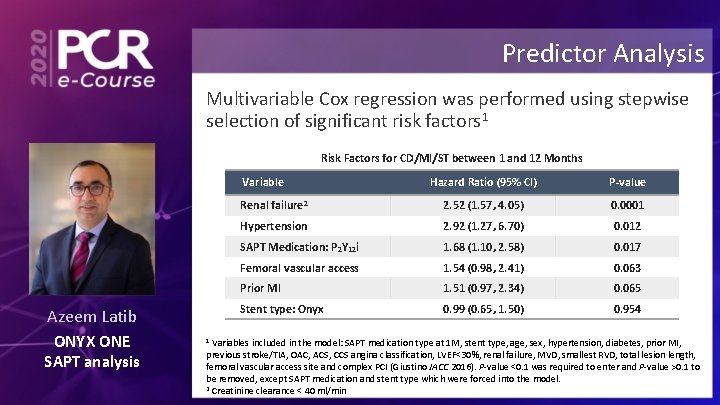
Predictor Analysis Multivariable Cox regression was performed using stepwise selection of significant risk factors 1 Risk Factors for CD/MI/ST between 1 and 12 Months Variable Azeem Latib ONYX ONE SAPT analysis Hazard Ratio (95% CI) P-value Renal failure 2 2. 52 (1. 57, 4. 05) 0. 0001 Hypertension 2. 92 (1. 27, 6. 70) 0. 012 SAPT Medication: P 2 Y 12 i 1. 68 (1. 10, 2. 58) 0. 017 Femoral vascular access 1. 54 (0. 98, 2. 41) 0. 063 Prior MI 1. 51 (0. 97, 2. 34) 0. 065 Stent type: Onyx 0. 99 (0. 65, 1. 50) 0. 954 Variables included in the model: SAPT medication type at 1 M, stent type, age, sex, hypertension, diabetes, prior MI, previous stroke/TIA, OAC, ACS, CCS angina classification, LVEF<30%, renal failure, MVD, smallest RVD, total lesion length, femoral vascular access site and complex PCI (Giustino JACC 2016). P-value <0. 1 was required to enter and P-value >0. 1 to be removed, except SAPT medication and stent type which were forced into the model. 2 Creatinine clearance < 40 ml/min 1

The essentials to remember • In the Onyx ONE trial, 92% were on SAPT at 2 months • 56% were taking aspirin monotherapy and 44% P 2 Y 12 i • Patients on P 2 Y 12 i were less often female, but had significantly more Afib, previous stroke and more HBR criteria than aspirin patients • Adverse ischemic and bleeding rates between 1 and 12 months were similar irrespective of the SAPT agent that was continued, except for cardiac death which was higher with P 2 Y 12 i Azeem Latib • Choice of SAPT agent was an independent predictor of CD/MI/ST between 1 and 12 months ONYX ONE SAPT analysis • Future analyses will expand the dataset with the Onyx ONE Clear study patients

PCRonline. com