Oncolytic Viruses already approved Two adenovirus particles Transmission





- Slides: 12

Oncolytic Viruses already approved Two adenovirus particles Transmission electron micrograph 1. Oncorine (H 101) & Onyx-15 The first oncolytic virus to be approved by a regulatory agency was a genetically modified adenovirus named H 101, produced by Shanghai Sunway Biotech. It gained regulatory approval in 2005 from China's State Food and Drug Administration (SFDA) for the treatment of head and neck cancer. Adenoviruses are medium-sized (90– 100 nm), non-enveloped (without an outer lipid bilayer) viruses with an icosahedral nucleocapsid containing a double stranded DNA genome. Their name derives from their initial isolation from human adenoids in 1953. In humans, more than 50 distinct adenoviral serotypes have been found to cause a wide range of illnesses. The early genes are responsible for expressing mainly non-structural, regulatory proteins. The E 1 A gene is responsible for inactivation of several proteins including retinoblastoma, allowing entry into S-phase. The E 1 B 55 k. Da gene cooperates with another adenoviral product, E 4 ORF 6, to inactivate p 53, thus preventing apoptosis. Sunway's H 101 and the very similar Onyx 15 have been engineered to remove the viral defense mechanism - E 1 B 55 k. Da gene - that interacts with the normal human gene p 53, which is very frequently dysregulated in cancer cells. H 101 is now marketed under the brand name Oncorine. Citations form a review asking “what have we learned from Clinical research results with dl 1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer? ”: “The virus was generally well-tolerated at doses of up to 2 × 1012 particles by intratumoral, intraperitoneal, hepatic arterial and intravenous administration. Viral replication was tumor-selective, and was documented (by immunohistochemistry) after administration by all routes. Replication was generally transient (<10 days), however, and was variable depending on tumor histology. Single agent efficacy has been limited to date (0– 14% local tumor regression rates). In combination with chemotherapy, however, encouraging antitumoral activity has been demonstrated… Future improvements with this approach will be possible if the reasons for dl 1520 failure as a single agent, and relative success in combination with chemotherapy, are uncovered… it is important to remember that this virus is attenuated relative to wild-type adenovirus in most tumor cell lines in vitro and in vivo, including even p 53 mutant tumors. This is not an unexpected phenotype since this virus has lost critical E 1 B-55 k. D functions that are unrelated to p 53, including viral m. RNA transport & host cell protein synthesis shut-off. . . Innate and acquired immune responses to the virus may be critical… Given the high degree of safety but inadequate single agent efficacy of dl 1520 against advanced solid tumors, second generation viruses will clearly be engineered for greater potency… by arming viruses with therapeutic genes (eg prodrug-activating enzymes… Finally, we must identify the mechanisms leading to the potential synergy between replicating adenoviral therapy and chemotherapy. This understanding may then allow us to bolster this interaction… Since intratumoral spread also appears to be a substantial hurdle for viral agents, inherently motile agents such as bacteria may hold great promise for this field. The clinical development of the first-generation adenovirus dl 1520 (Onyx-015) has taught us a great deal about the hurdles to be overcome with the replication-selective adenovirus approach. However, this novel therapeutic platform clearly has great potential to improve and prolong the lives of cancer patients.

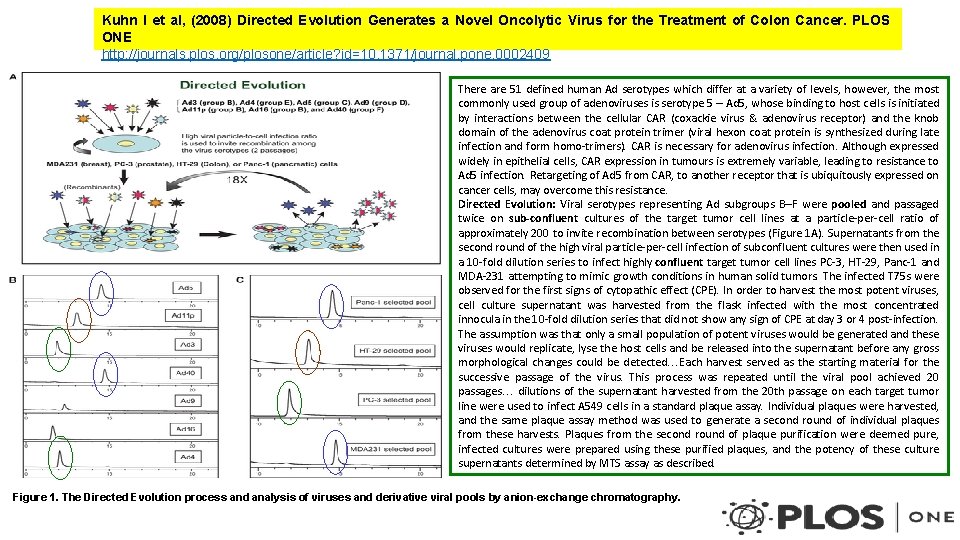
Kuhn I et al, (2008) Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLOS ONE http: //journals. plos. org/plosone/article? id=10. 1371/journal. pone. 0002409 There are 51 defined human Ad serotypes which differ at a variety of levels, however, the most commonly used group of adenoviruses is serotype 5 – Ad 5, whose binding to host cells is initiated by interactions between the cellular CAR (coxackie virus & adenovirus receptor) and the knob domain of the adenovirus coat protein trimer (viral hexon coat protein is synthesized during late infection and form homo-trimers). CAR is necessary for adenovirus infection. Although expressed widely in epithelial cells, CAR expression in tumours is extremely variable, leading to resistance to Ad 5 infection. Retargeting of Ad 5 from CAR, to another receptor that is ubiquitously expressed on cancer cells, may overcome this resistance. Directed Evolution: Viral serotypes representing Ad subgroups B–F were pooled and passaged twice on sub-confluent cultures of the target tumor cell lines at a particle-per-cell ratio of approximately 200 to invite recombination between serotypes (Figure 1 A). Supernatants from the second round of the high viral particle-per-cell infection of subconfluent cultures were then used in a 10 -fold dilution series to infect highly confluent target tumor cell lines PC-3, HT-29, Panc-1 and MDA-231 attempting to mimic growth conditions in human solid tumors. The infected T 75 s were observed for the first signs of cytopathic effect (CPE). In order to harvest the most potent viruses, cell culture supernatant was harvested from the flask infected with the most concentrated innocula in the 10 -fold dilution series that did not show any sign of CPE at day 3 or 4 post-infection. The assumption was that only a small population of potent viruses would be generated and these viruses would replicate, lyse the host cells and be released into the supernatant before any gross morphological changes could be detected…Each harvest served as the starting material for the successive passage of the virus. This process was repeated until the viral pool achieved 20 passages… dilutions of the supernatant harvested from the 20 th passage on each target tumor line were used to infect A 549 cells in a standard plaque assay. Individual plaques were harvested, and the same plaque assay method was used to generate a second round of individual plaques from these harvests. Plaques from the second round of plaque purification were deemed pure, infected cultures were prepared using these purified plaques, and the potency of these culture supernatants determined by MTS assay as described. Figure 1. The Directed Evolution process and analysis of viruses and derivative viral pools by anion-exchange chromatography.

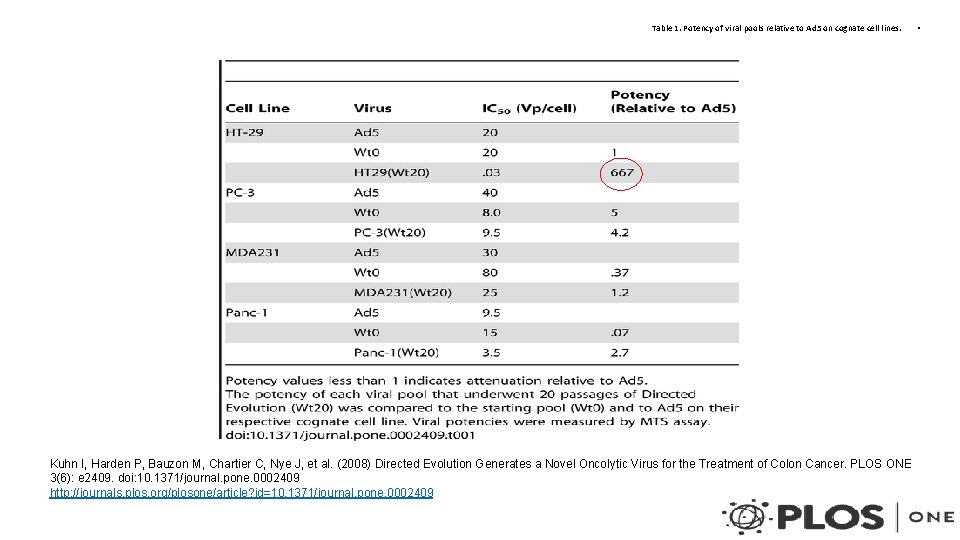
Table 1. Potency of viral pools relative to Ad 5 on cognate cell lines. Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, et al. (2008) Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLOS ONE 3(6): e 2409. doi: 10. 1371/journal. pone. 0002409 http: //journals. plos. org/plosone/article? id=10. 1371/journal. pone. 0002409 •

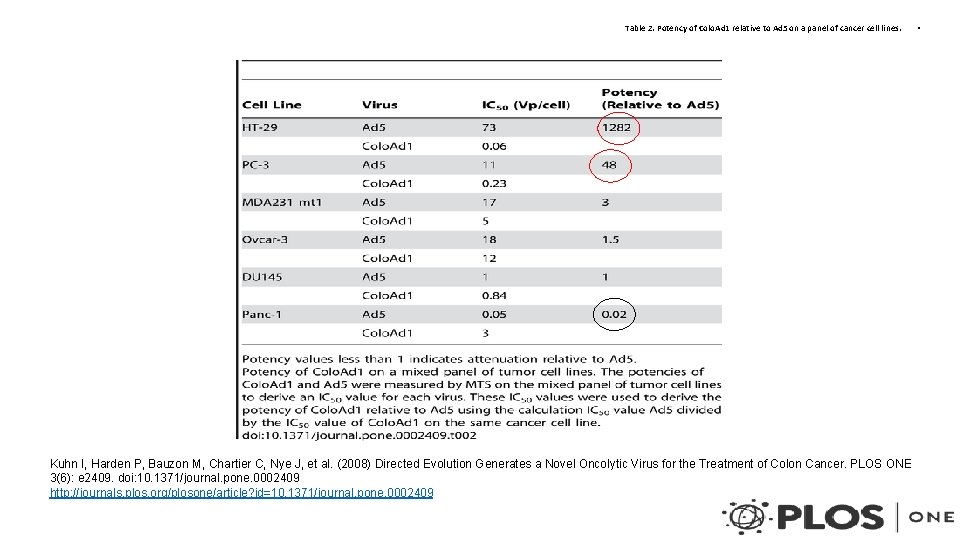
Table 2. Potency of Colo. Ad 1 relative to Ad 5 on a panel of cancer cell lines. Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, et al. (2008) Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLOS ONE 3(6): e 2409. doi: 10. 1371/journal. pone. 0002409 http: //journals. plos. org/plosone/article? id=10. 1371/journal. pone. 0002409 •

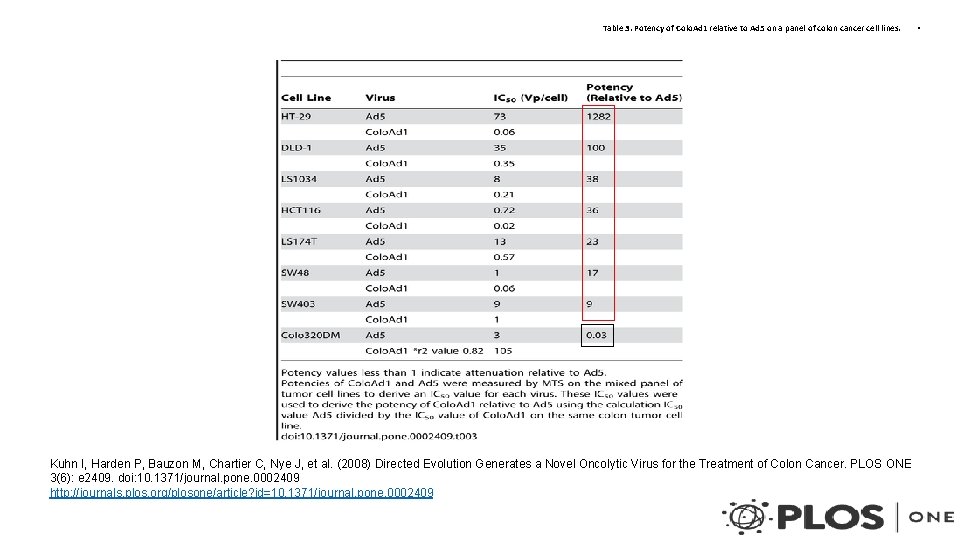
Table 3. Potency of Colo. Ad 1 relative to Ad 5 on a panel of colon cancer cell lines. Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, et al. (2008) Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLOS ONE 3(6): e 2409. doi: 10. 1371/journal. pone. 0002409 http: //journals. plos. org/plosone/article? id=10. 1371/journal. pone. 0002409 •

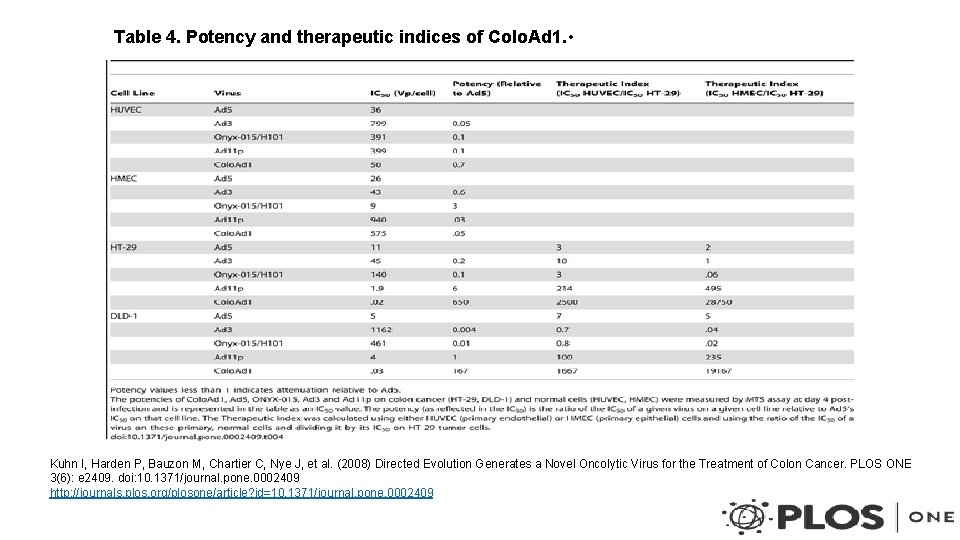
Table 4. Potency and therapeutic indices of Colo. Ad 1. • Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, et al. (2008) Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLOS ONE 3(6): e 2409. doi: 10. 1371/journal. pone. 0002409 http: //journals. plos. org/plosone/article? id=10. 1371/journal. pone. 0002409

Figure 2. Genomic sequence diagram of Colo. Ad 1 To determine the relationship between Colo. Ad 1 and Ad 11 p, the virus was sequenced, revealing that Colo. Ad 1 is Ad 11 p, with a nearly complete E 3 region deletion, a smaller deletion in the E 4 region, and a chimeric Ad 3/Ad 11 p E 2 B region Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, et al. (2008) Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLOS ONE 3(6): e 2409. doi: 10. 1371/journal. pone. 0002409 http: //journals. plos. org/plosone/article? id=10. 1371/journal. pone. 0002409

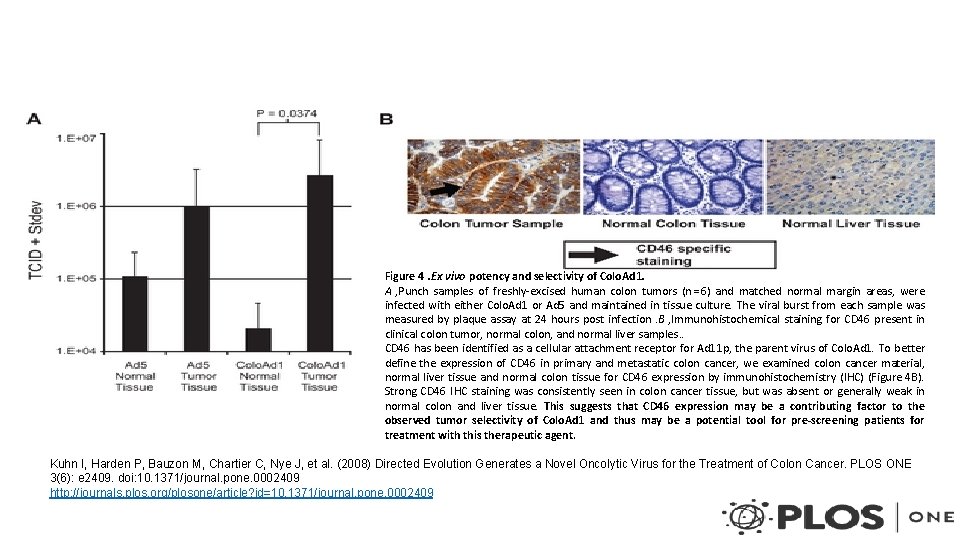
Figure 4. Ex vivo potency and selectivity of Colo. Ad 1. A , Punch samples of freshly-excised human colon tumors (n = 6) and matched normal margin areas, were infected with either Colo. Ad 1 or Ad 5 and maintained in tissue culture. The viral burst from each sample was measured by plaque assay at 24 hours post infection. B , Immunohistochemical staining for CD 46 present in clinical colon tumor, normal colon, and normal liver samples. . CD 46 has been identified as a cellular attachment receptor for Ad 11 p, the parent virus of Colo. Ad 1. To better define the expression of CD 46 in primary and metastatic colon cancer, we examined colon cancer material, normal liver tissue and normal colon tissue for CD 46 expression by immunohistochemistry (IHC) (Figure 4 B). Strong CD 46 IHC staining was consistently seen in colon cancer tissue, but was absent or generally weak in normal colon and liver tissue. This suggests that CD 46 expression may be a contributing factor to the observed tumor selectivity of Colo. Ad 1 and thus may be a potential tool for pre-screening patients for treatment with this therapeutic agent. Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, et al. (2008) Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLOS ONE 3(6): e 2409. doi: 10. 1371/journal. pone. 0002409 http: //journals. plos. org/plosone/article? id=10. 1371/journal. pone. 0002409

From the discussion: This virus has several changes relative to the parent Ad 11 p virus, including a chimeric E 2 B region and deletions in the E 3 and E 4 regions… The loss of genes in the Ad 5 E 3 region has been shown in group B Ads to enhance viral lysis and spread by an undefined mechanism. The E 2 B region encodes the pre-terminal protein (p. TP) and the viral DNA polymerase (DNA pol), two of the three E 2 -encoded proteins necessary for viral DNA replication. The terminal 18 bp of the viral genome, considered the minimal replication origin, directly interacts with the p. TP and DNA pol heterodimer… Thus, the E 2 B alterations in Colo. Ad 1 may generate a p. TP-DNA pol heterodimer that is more compatible with the terminal 18 bp of Colo. Ad 1 than the original Ad 11 p p. TP-DNA pol. Coupled with Colo. Ad 1’s smaller genome (a result of genomic deletions) this virus may replicate more quickly and also reach a critical viral burst size more rapidly, consequently enhancing viral lysis and spread. With regard to selectivity, studies with the p. TP of Ad 5 have shown that it interacts with CAD, a host protein responsible for the TP-nuclear matrix association. The level of CAD is correlated with the rate of cell division; two to five-fold higher levels in tumor cells than in normal cells and almost nonexistent in quiescent cells. Consequently, TP alterations and their potential interactions with CAD (or similar proteins) may also be mechanisms of enhanced replication and/or selectivity of the virus… It is important to note that Colo. Ad 1 is a member of the group B Ads and thus distinctly different from the traditional Ad 5 -based oncolytic viruses. It does not, for example, use the Ad 5 receptor, (coxsackie B- and adenovirus receptor, CAR), for attachment to cells. Instead, Colo. Ad 1 appears to employ at least two receptors that are distinctly different from CAR, one of which has been recently described as CD 46. The significance of this is emphasized by recent studies on clinical material showing that CAR is poorly expressed on a variety of different tumor types and that CAR expression decreases with the advance in stage and grade of the tumor. . . In contrast, tumor cell surface expression of CD 46, the putative Colo. Ad 1 receptor, appears to increase with stage and grade in a variety of cancers. Thus, Colo. Ad 1 may have therapeutic utility beyond colon cancer, and studies to investigate this are ongoing. Of additional importance are data demonstrating that the seroprevalence (the number of persons in a population who test positive for a specific disease based on serology [blood serum] specimens… Positively identifying the occurrence of disease is usually based upon the presence of antibodies for that disease) of Ad 11 p is low. Since the greatest need for this type of therapeutic is in patient populations where the tumor has progressed from a confined local disease to a systemic, metastatic cancer, treating patients with an agent to which they do not have pre-existing immunity should enhance the opportunity for the agent to circulate and eliminate metastatic tumor cells. This is in contrast to Ad 5 where seroprevalence, as measured by neutralizing antibodies, reaches levels of approximately 50% in the general population

2. Rigvir: Enteroviruses are a genus of positive-sense single-stranded RNA viruses associated with several human and mammalian diseases. Enteroviruses are named by their transmission-route through the intestine (enteric meaning intestinal). Enteroviruses affect millions of people worldwide each year, and are often found in the respiratory secretions (e. g. , saliva, sputum, or nasal mucus) and stool of an infected person. Poliomyelitis was the most significant disease caused by an enterovirus the poliovirus. • In 1960 unknown phenomenon was discovered: when human enteric viruses (enteroviruses of group ECHO), which were isolated from the gastrointestinal tract of young children and grafted into hamster buccal bags showed oncotropic and oncolytic properties. • In 1965, cancer virotherapy laboratory was founded… 60 different types of enteric viruses were tested… One of them, ECHO-7, was named Rigvir (Riga virus). It was found that Rigvir is safe for adults and not able to replicate in the human body. • In 1968 scientists received permission from the Ministry of Health of the Latvian SSR to study and use Rigvir on patients… • In 1985, received approval from the All-Union Cancer Center in Moscow… • In 1990– 1995, Rigvir used to treat patients with various kinds of cancer in the P. Stradina clinic and Latvian Oncology Center… • On October 20, 2002 a patent was granted for Rigvir • On April 29, 2004 Rigvir was approved by the State Agency of Medicines of the Republic of Latvia. • In February 2015 registered in Georgia. • In 2015 Rigvir was included in Latvian clinical guidlines for skin cancer and melanoma, published by National Health Service of Latvia. Page 11 specifies that, according to the criteria of European Society for Medical Oncology outlined in pages 7– 8, evidence level for Rigvir even in the case of skin melanoma (which is the only indication Rigvir is registered for) is III/C, meaning: "insufficient evidence of efficacy, or benefits do not exceed disadvantages (negative outcomes, cost of treatment and other risks); optional use"… • In 2016 RIGVIR was approved in Armenia. Virions of enteroviruses in Rigvir

3. Talimogen laherparepvec, Onco. VEX GM-CSF, T-vec, by Amgen, successfully completed phase III trials for advanced melanoma in March 2013. In October 2015, the US FDA approved T-VEC, with the brand name Imlygic , for the treatment of melanoma in patients with inoperable tumors becoming the first approved oncolytic agent in the western world. It is based on herpes simplex virus (HSV-1). It has also been tested in a Phase I trial for pancreatic cancer and a Phase III trial in head and neck cancer together with chemotherapy and radiotherapy. Herpesviridae is a large family of DNA viruses that cause diseases in animals, including humans. The members of this family are also known as herpesviruses. TEM micrograph of a herpes simplex virus 3 D reconstruction of the Herpes simplex virus type 1 (HSV-1) capsid

ICP 34. 5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. BL Liu et al. Gene Therapy (2003) 10, 292– 303 “In this paper, we have built on previous work to improve the potency of oncolytic HSV. We aimed to boost both the tumour-selective replicative capability of the virus and also its immune stimulating ability to provide a multi-modal cancer therapy incorporating both direct virus replication-mediated cell lysis and the induction of an immune response to the antigens released. Such a virus would, apart from having a direct oncolytic effect, provide an in situ, patient-specific, antitumour vaccine…In order to develop oncolytic HSV with greater tumour-selective replicative ability, we tested two clinical isolates of HSV for their ability to replicate in and kill human tumour cell lines. It was anticipated that recent clinical isolates might be more effective than the previously tested serially passaged laboratory strains. This proved to be the case, and the recent clinical strain of HSV 1, JS 1, was chosen for further development. The genetic basis of the improved cell killing properties of JS 1 as compared to the reference strain (strain 17+) has not been fully explored…We deleted the genes encoding ICP 34. 5 to provide tumour-selective replication, and then also included a previously reported mutation resulting in the expression of the US 11 gene as an IE rather than on L gene to further increase replication. Expression of US 11 blocks phosphorylation of PKR which results in enhanced replication of ICP 34. 5 deleted viruses in tumour cells without compromising safety. When tested in mouse xenograft tumour models, this mutation considerably enhanced the anti-tumour effect, as expected from previous work. Increased expression of US 11 is accomplished by deletion of the coding sequence for ICP 47. Since ICP 47 usually inhibits antigen presentation in HSV infected cells, this deletion would be expected to increase levels of antigen presentation in infected tumour cells. This should improve any anti-tumour immune response following intra-tumoral injection of the virus…tumour antigens would be expected to be expressed more effectively on the surface of infected tumour cells prior to cell death… As expected, we observed increased class I MHC expression on the surface of human cells infected with JS 1/ICP 34. 5/ICP 47, compared to the equivalent virus in which ICP 34. 5 alone was deleted… Finally, to improve the potential for an anti-tumour immune response further, we inserted the gene for GMCSF. While a number of cytokines have been considered for use in anti-tumour therapy, GM-CSF has generally, and most reliably, given the best results when tested in comparison to other cytokines in pre-clinical models. . . When the gene for GM-CSF was inserted into the ICP 34. 5/ICP 47 deleted version of JS 1, an enhanced antitumour effect was seen in artificially induced tumours in immune competent mice where significant effects on both injected and uninjected tumours were seen. Mice were also then protected from re-challenge with tumour cells. An enhanced tumour-specific immune response with the virus encoding GM-CSF was also demonstrated. Overall therefore, we describe a highly potent oncolytic version of HSV which is designed to also stimulate an enhanced anti-tumour immune response by the inclusion of the gene encoding GM-CSF. The virus would therefore be anticipated to have a potent oncolytic anti-tumour effect if used for cancer treatment in man, and also provide an in situ, patient-specific tumour vaccine, following intratumoral injection and the liberation of tumour antigens. The virus should therefore have effects on both infected and uninfected tumors… “
 Lytic cycle animation
Lytic cycle animation /watch?v=dckvspcd8gs
/watch?v=dckvspcd8gs Already can or can already
Already can or can already Sklerada
Sklerada Life cycle of adenovirus
Life cycle of adenovirus John is over two hours late already
John is over two hours late already Relative motion of two particles using translating axes
Relative motion of two particles using translating axes Position and motion
Position and motion Why are viruses considered nonliving?
Why are viruses considered nonliving? Bacteriophage characteristics
Bacteriophage characteristics General characteristics of viruses
General characteristics of viruses Viruses
Viruses Lysogenic viruses do not
Lysogenic viruses do not