OECD PRINCIPLES OF GLP AND TEST GUIDELINES EU





- Slides: 7

OECD PRINCIPLES OF GLP AND TEST GUIDELINES EU Parliament’s Special Committee on the Union’s authorisation procedure for pesticides Bob Diderich, ENV/EHS, OECD

Problem Formulation • More than 100 000 chemicals on the markets of OECD countries • About 1000 new chemicals every year • Ensuring the safe use of those chemicals is very resource intensive for both industry and governments • Harmonising the risk assessment methodologies allows for “sharing the burden” between countries 2

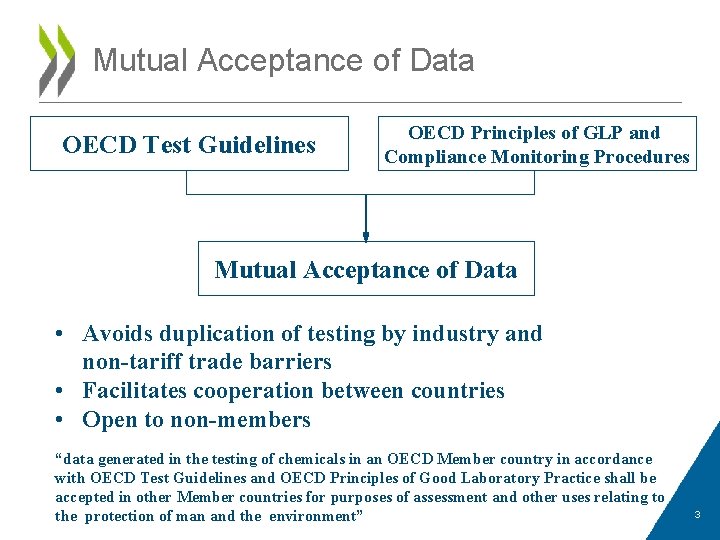
Mutual Acceptance of Data OECD Test Guidelines OECD Principles of GLP and Compliance Monitoring Procedures Mutual Acceptance of Data • Avoids duplication of testing by industry and non-tariff trade barriers • Facilitates cooperation between countries • Open to non-members “data generated in the testing of chemicals in an OECD Member country in accordance with OECD Test Guidelines and OECD Principles of Good Laboratory Practice shall be accepted in other Member countries for purposes of assessment and other uses relating to the protection of man and the environment” 3

OECD Test Guidelines • A collection of the most relevant internationally agreed test methods that cover tests for: 1. 2. 3. 4. 5. Physical Chemical Properties Effects on Biotic Systems Degradation and Accumulation Health Effects Other TGs (Pesticide Residue Chemistry, TGs for biocide efficacy) • Currently there are over 150 OECD Test Guidelines 4

OECD Principles of Good Laboratory Practice • Good Laboratory Practice (GLP) refers to a quality system of management controls for research laboratories and organizations conducting non-clinical health and safety tests. • GLP ensures the uniformity, consistency, reliability, reproducibility, quality, and integrity of chemical tests.

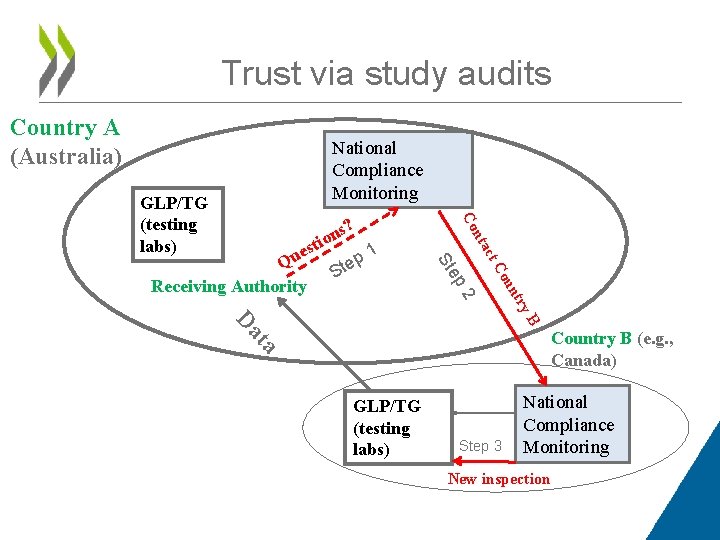
Trust via study audits Country A (Australia) National Compliance Monitoring tac try 2 n ou t. C ep St ns? o i 1 est u p Q e St Receiving Authority n Co GLP/TG (testing labs) B ta Da Country B (e. g. , Canada) GLP/TG (testing labs) Step 3 National Compliance Monitoring New inspection

Scope • “Non-clinical” testing of a chemical or chemical product under laboratory conditions or in the environment • Products: Ø Ø Ø Industrial chemicals Pharmaceuticals Pesticides Biocides Veterinary drugs Food and feed additives 7