NORTHWEST AIDS EDUCATION AND TRAINING CENTER Crafting an









- Slides: 18

NORTHWEST AIDS EDUCATION AND TRAINING CENTER Crafting an ART Regimen for Initiation or Salvage: Are NRTI’s Necessary? Brian R. Wood, MD Assistant Professor of Medicine, University of Washington Medical Director, NW AETC ECHO Last Updated: 1/22/15

NRTI-Sparing Regimens: Outline • • Treatment initiation Switching for maintenance Salvage therapy Future questions and directions


Why Consider NRTI-Sparing Regimens? • NRTI’s are the backbone of first-line and salvage regimens • However, toxicity can be limiting - Tenofovir renal and bone effects - Abacavir hypersensitivity if B*5701(+), ? CV effects - Older NRTI’s many short and long-term side effects • Resistance may preclude use

NRTI SPARING-REGIMENS Data for Use as Initial Therapy

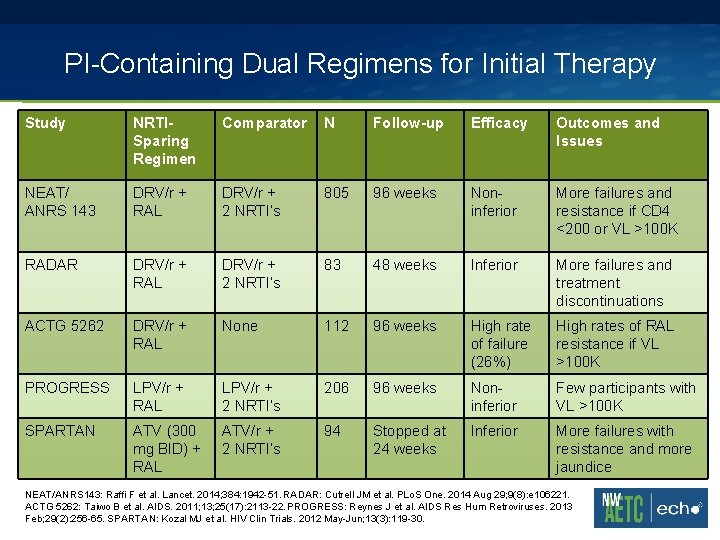
PI-Containing Dual Regimens for Initial Therapy Study NRTISparing Regimen Comparator N Follow-up Efficacy Outcomes and Issues NEAT/ ANRS 143 DRV/r + RAL DRV/r + 2 NRTI’s 805 96 weeks Noninferior More failures and resistance if CD 4 <200 or VL >100 K RADAR DRV/r + RAL DRV/r + 2 NRTI’s 83 48 weeks Inferior More failures and treatment discontinuations ACTG 5262 DRV/r + RAL None 112 96 weeks High rate of failure (26%) High rates of RAL resistance if VL >100 K PROGRESS LPV/r + RAL LPV/r + 2 NRTI’s 206 96 weeks Noninferior Few participants with VL >100 K SPARTAN ATV (300 mg BID) + RAL ATV/r + 2 NRTI’s 94 Stopped at 24 weeks Inferior More failures with resistance and more jaundice NEAT/ANRS 143: Raffi F et al. Lancet. 2014; 384: 1942 -51. RADAR: Cutrell JM et al. PLo. S One. 2014 Aug 29; 9(8): e 106221. ACTG 5262: Taiwo B et al. AIDS. 2011; 13; 25(17): 2113 -22. PROGRESS: Reynes J et al. AIDS Res Hum Retroviruses. 2013 Feb; 29(2): 256 -65. SPARTAN: Kozal MJ et al. HIV Clin Trials. 2012 May-Jun; 13(3): 119 -30.

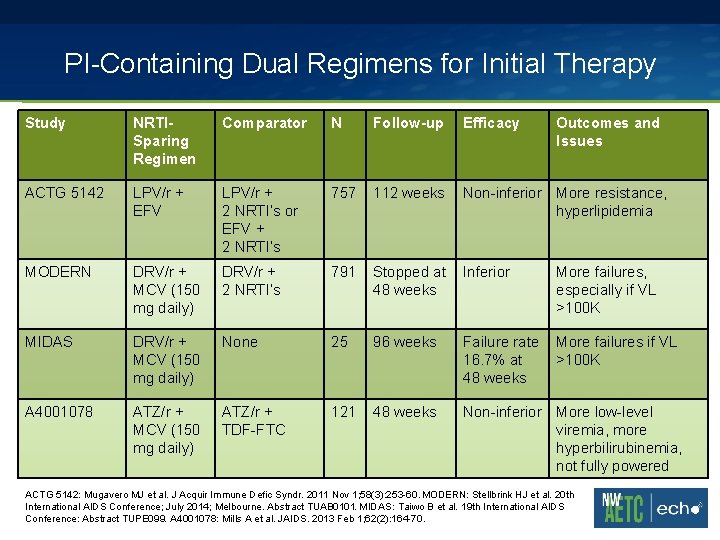
PI-Containing Dual Regimens for Initial Therapy Study NRTISparing Regimen Comparator N Follow-up Efficacy Outcomes and Issues ACTG 5142 LPV/r + EFV LPV/r + 2 NRTI’s or EFV + 2 NRTI’s 757 112 weeks Non-inferior More resistance, hyperlipidemia MODERN DRV/r + MCV (150 mg daily) DRV/r + 2 NRTI’s 791 Stopped at 48 weeks Inferior More failures, especially if VL >100 K MIDAS DRV/r + MCV (150 mg daily) None 25 96 weeks Failure rate 16. 7% at 48 weeks More failures if VL >100 K A 4001078 ATZ/r + MCV (150 mg daily) ATZ/r + TDF-FTC 121 48 weeks Non-inferior More low-level viremia, more hyperbilirubinemia, not fully powered ACTG 5142: Mugavero MJ et al. J Acquir Immune Defic Syndr. 2011 Nov 1; 58(3): 253 -60. MODERN: Stellbrink HJ et al. 20 th International AIDS Conference; July 2014; Melbourne. Abstract TUAB 0101. MIDAS: Taiwo B et al. 19 th International AIDS Conference: Abstract TUPE 099. A 4001078: Mills A et al. JAIDS. 2013 Feb 1; 62(2): 164 -70.

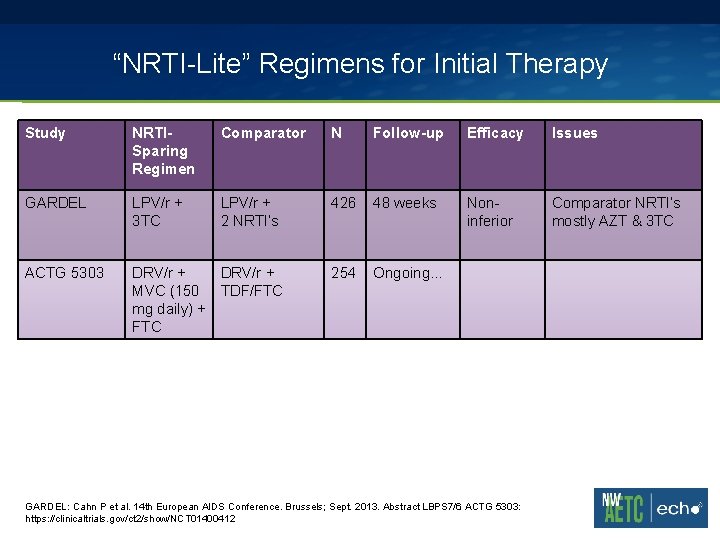
“NRTI-Lite” Regimens for Initial Therapy Study NRTISparing Regimen Comparator N Follow-up Efficacy Issues GARDEL LPV/r + 3 TC LPV/r + 2 NRTI’s 426 48 weeks Noninferior Comparator NRTI’s mostly AZT & 3 TC ACTG 5303 DRV/r + MVC (150 TDF/FTC mg daily) + FTC 254 Ongoing… GARDEL: Cahn P et al. 14 th European AIDS Conference. Brussels; Sept. 2013. Abstract LBPS 7/6. ACTG 5303: https: //clinicaltrials. gov/ct 2/show/NCT 01400412

NRTI-Sparing or “Lite” Regimens for Initial Therapy: Summary • Studies limited by unusual dosing, outdated comparators, insufficient power, and other issues • Most studies show lower efficacy or more side effects without improving pill burden or dosing frequency • Two trials with the most reassuring results used boosted lopinavir, which is no longer a recommended agent • Need well-designed trials of modern drugs! - ie. boosted darunavir + dolutegravir +/- 3 TC/FTC

NRTI SPARING-REGIMENS Data for Use as Maintenance Therapy

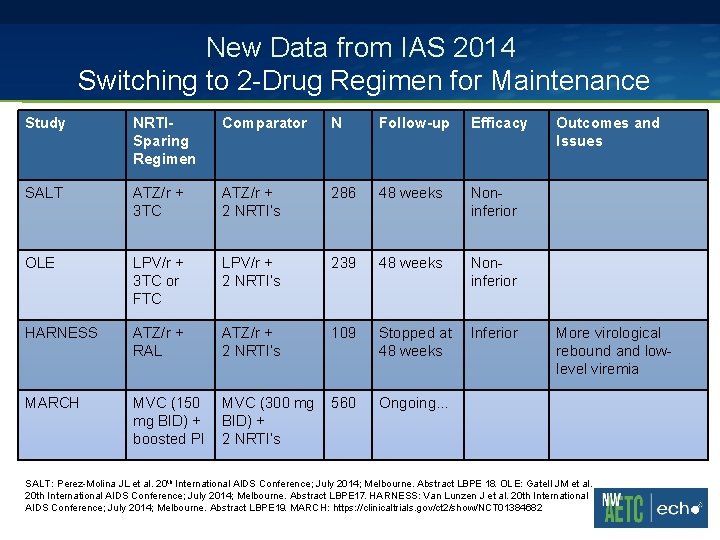
New Data from IAS 2014 Switching to 2 -Drug Regimen for Maintenance Study NRTISparing Regimen Comparator N Follow-up Efficacy SALT ATZ/r + 3 TC ATZ/r + 2 NRTI’s 286 48 weeks Noninferior OLE LPV/r + 3 TC or FTC LPV/r + 2 NRTI’s 239 48 weeks Noninferior HARNESS ATZ/r + RAL ATZ/r + 2 NRTI’s 109 Stopped at 48 weeks Inferior MARCH MVC (150 mg BID) + boosted PI MVC (300 mg BID) + 2 NRTI’s 560 Ongoing… Outcomes and Issues More virological rebound and lowlevel viremia SALT: Perez-Molina JL et al. 20 th International AIDS Conference; July 2014; Melbourne. Abstract LBPE 18. OLE: Gatell JM et al. 20 th International AIDS Conference; July 2014; Melbourne. Abstract LBPE 17. HARNESS: Van Lunzen J et al. 20 th International AIDS Conference; July 2014; Melbourne. Abstract LBPE 19. MARCH: https: //clinicaltrials. gov/ct 2/show/NCT 01384682

NRTI SPARING-REGIMENS Data for Use as Salvage Therapy

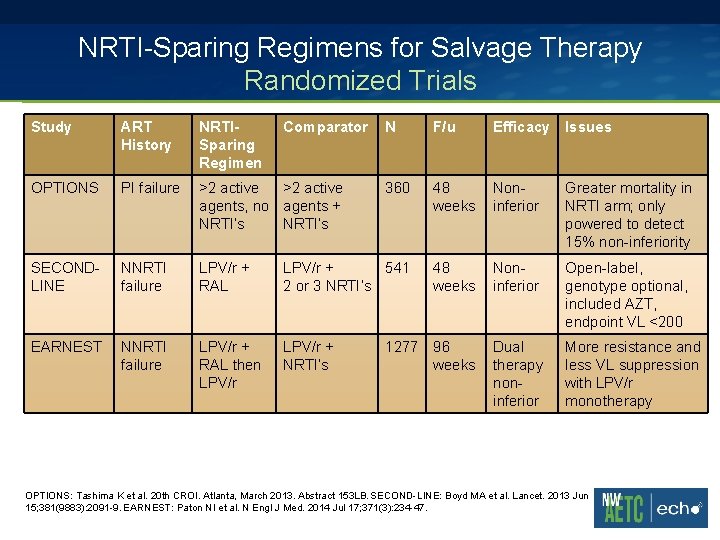
NRTI-Sparing Regimens for Salvage Therapy Randomized Trials Study ART History NRTISparing Regimen Comparator N F/u Efficacy Issues OPTIONS PI failure >2 active agents, no agents + NRTI’s 360 48 weeks Noninferior Greater mortality in NRTI arm; only powered to detect 15% non-inferiority SECONDLINE NNRTI failure LPV/r + RAL LPV/r + 541 2 or 3 NRTI’s 48 weeks Noninferior Open-label, genotype optional, included AZT, endpoint VL <200 EARNEST NNRTI failure LPV/r + RAL then LPV/r + NRTI’s 1277 96 weeks Dual therapy noninferior More resistance and less VL suppression with LPV/r monotherapy OPTIONS: Tashima K et al. 20 th CROI. Atlanta, March 2013. Abstract 153 LB. SECOND-LINE: Boyd MA et al. Lancet. 2013 Jun 15; 381(9883): 2091 -9. EARNEST: Paton NI et al. N Engl J Med. 2014 Jul 17; 371(3): 234 -47.

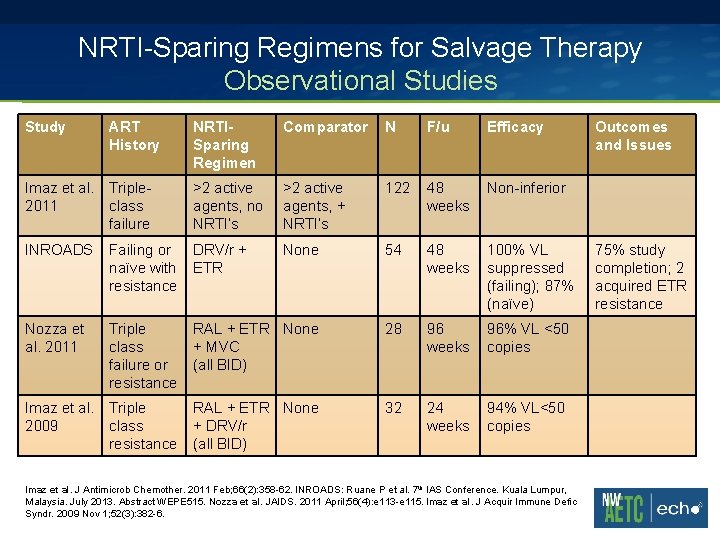
NRTI-Sparing Regimens for Salvage Therapy Observational Studies Study ART History NRTISparing Regimen Comparator N F/u Efficacy Imaz et al. Triple 2011 class failure >2 active agents, no NRTI’s >2 active agents, + NRTI’s 122 48 weeks Non-inferior INROADS Failing or naïve with resistance DRV/r + ETR None 54 48 weeks 100% VL suppressed (failing); 87% (naïve) Nozza et al. 2011 Triple class failure or resistance RAL + ETR None + MVC (all BID) 28 96 weeks 96% VL <50 copies Imaz et al. Triple 2009 class resistance RAL + ETR None + DRV/r (all BID) 32 24 weeks 94% VL<50 copies Imaz et al. J Antimicrob Chemother. 2011 Feb; 66(2): 358 -62. INROADS: Ruane P et al. 7 th IAS Conference. Kuala Lumpur, Malaysia. July 2013. Abstract WEPE 515. Nozza et al. JAIDS. 2011 April; 56(4): e 113 -e 115. Imaz et al. J Acquir Immune Defic Syndr. 2009 Nov 1; 52(3): 382 -6. Outcomes and Issues 75% study completion; 2 acquired ETR resistance

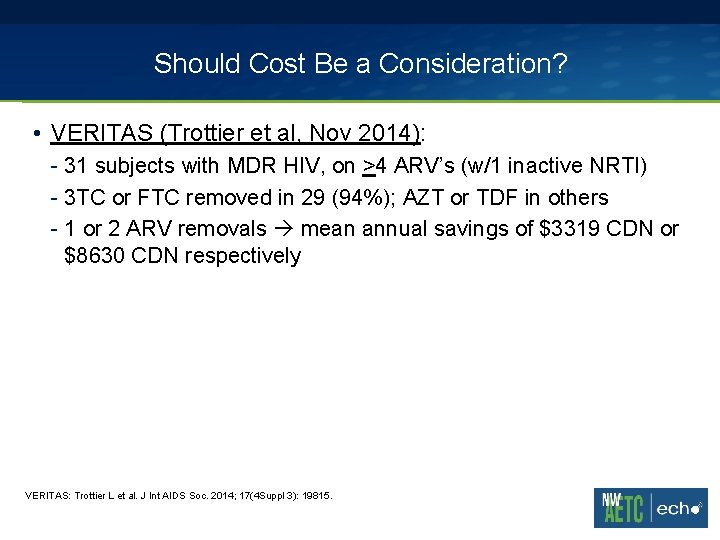
Should Cost Be a Consideration? • VERITAS (Trottier et al, Nov 2014): - 31 subjects with MDR HIV, on >4 ARV’s (w/1 inactive NRTI) - 3 TC or FTC removed in 29 (94%); AZT or TDF in others - 1 or 2 ARV removals mean annual savings of $3319 CDN or $8630 CDN respectively VERITAS: Trottier L et al. J Int AIDS Soc. 2014; 17(4 Suppl 3): 19815.

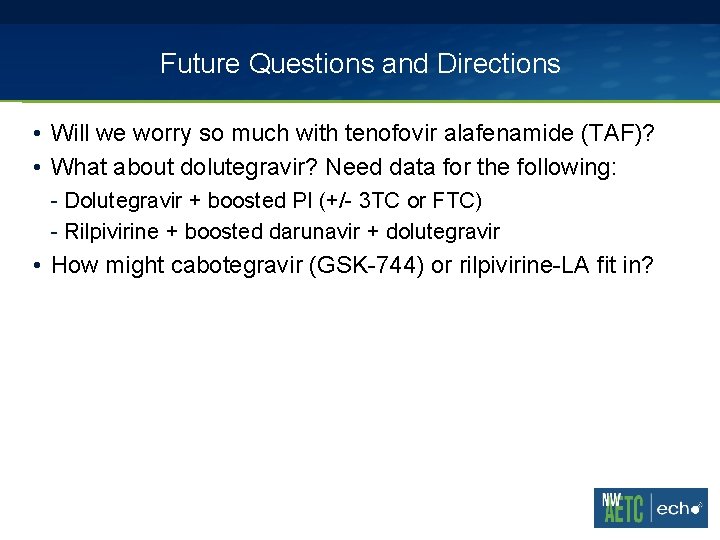
Future Questions and Directions • Will we worry so much with tenofovir alafenamide (TAF)? • What about dolutegravir? Need data for the following: - Dolutegravir + boosted PI (+/- 3 TC or FTC) - Rilpivirine + boosted darunavir + dolutegravir • How might cabotegravir (GSK-744) or rilpivirine-LA fit in?

NRTI-Sparing Regimens Take Home Points • Most data for initial therapy is limited by design/dosing issues • Dual therapy options should be used only in unique cases and perhaps for maintenance in select patients • Anecdotally, “NRTI-lite” regimens like 3 TC/FTC + boosted PI + integrase seem to work well, but we need data • More advanced HIV disease equates to higher risk of failure • Could consider including NRTI’s for salvage, at least until suppressed, then simplify

Case Question • A patient previously treated with multiple NRTI’s, efavirenz, and boosted lopinavir transfers care to you. He has been off ART and viral load is 9, 400. Prior genotypes show K 103 N, E 138 A, M 184 V, M 41 L, T 215 Y, K 219 Q, and PI mutations (but no darunavir-associated mutations). • He has never taken integrase inhibitors. You plan to restart ART with boosted darunavir, etravirine, and dolutegravir. • Would you add lamivudine (3 TC) or emtricitabine (FTC)? A) Yes, would add indefinitely B) Yes, would add until viral load suppressed then withdraw C) No, would not add
 Northwest regional data center
Northwest regional data center Nisd gala
Nisd gala Process of crafting and executing strategy
Process of crafting and executing strategy Potty training underware
Potty training underware Types of training aids
Types of training aids Crafting the service environment
Crafting the service environment Crafting a compiler
Crafting a compiler Crafting media messages
Crafting media messages Bull's eye brand positioning
Bull's eye brand positioning Service escape
Service escape Compositional mode for social media
Compositional mode for social media Crafting the brand positioning
Crafting the brand positioning Craft a personal entrepreneurial strategy
Craft a personal entrepreneurial strategy Research sub questions
Research sub questions Crafting a compiler
Crafting a compiler Crafting the service environment
Crafting the service environment Mountain dew positioning statement
Mountain dew positioning statement Crafting media messages
Crafting media messages Crafting a persuasive strategy presentation
Crafting a persuasive strategy presentation



































