Micro Vention New Products The CASPER device is




- Slides: 10

Micro. Vention New Products

The CASPER device is not cleared/approved by the U. S. FDA for sale or use in the United States.

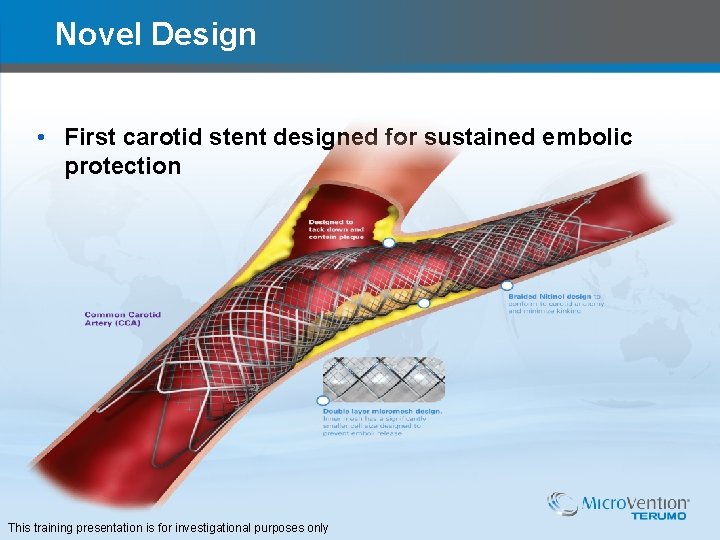
Novel Design • First carotid stent designed for sustained embolic protection This training presentation is for investigational purposes only

CASPER Carotid Stent • Sustained Embolic Protection – Dual layer micromesh design – Smallest stent cell size, ¼ size compared to smallest on market – Designed to prevent plaque prolapse • Braided Nitinol Design – Closed cell design with open cell mechanical performance – Optimal conformability and wall apposition – In-vivo tapering, less configurations to stock • Low Profile Delivery System – 5. 2 Fr. Rapid Exchange for all sizes – Up to 50% deployment • Fully re-sheathable • Fully repositionable Caution: The Casper device is not cleared/approved by the U. S. FDA for sale or use in the United States

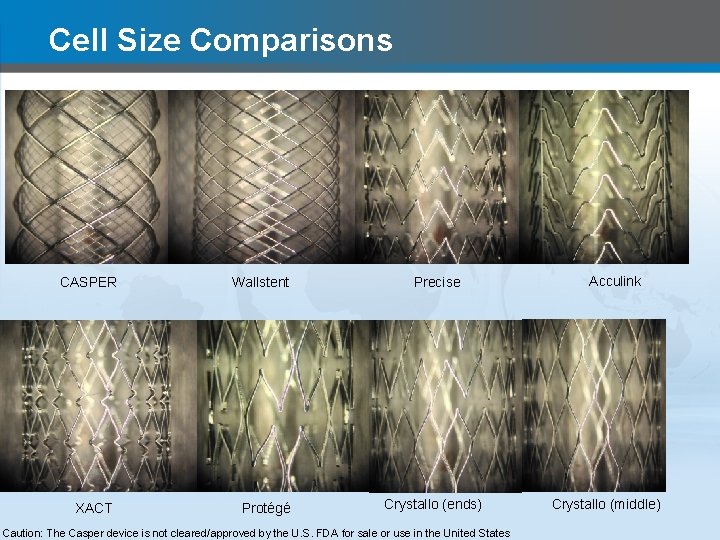
Cell Size Comparisons CASPER XACT Wallstent Protégé Precise Crystallo (ends) Caution: The Casper device is not cleared/approved by the U. S. FDA for sale or use in the United States Acculink Crystallo (middle)

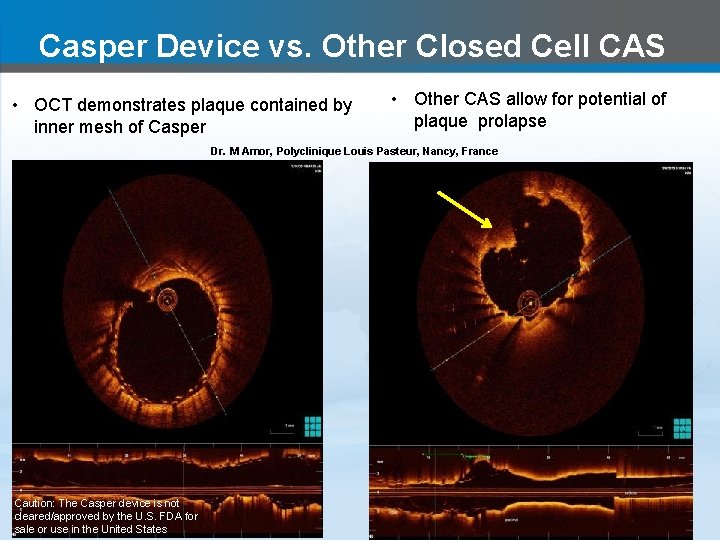
Casper Device vs. Other Closed Cell CAS • OCT demonstrates plaque contained by inner mesh of Casper • Other CAS allow for potential of plaque prolapse Dr. M Amor, Polyclinique Louis Pasteur, Nancy, France Caution: The Casper device is not cleared/approved by the U. S. FDA for sale or use in the United States

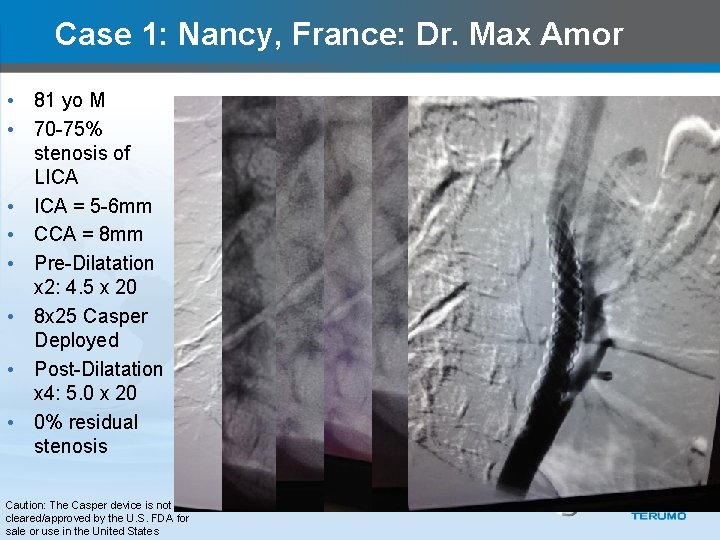
Case 1: Nancy, France: Dr. Max Amor • 81 yo M • 70 -75% stenosis of LICA • ICA = 5 -6 mm • CCA = 8 mm • Pre-Dilatation x 2: 4. 5 x 20 • 8 x 25 Casper Deployed • Post-Dilatation x 4: 5. 0 x 20 • 0% residual stenosis Caution: The Casper device is not cleared/approved by the U. S. FDA for sale or use in the United States

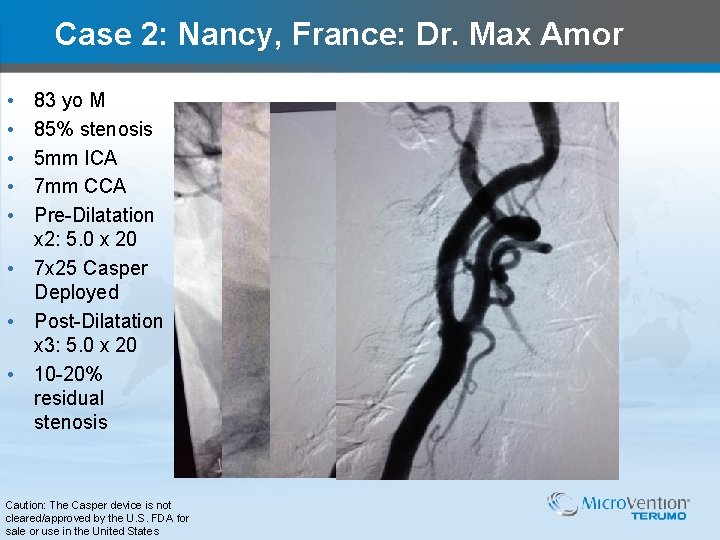
Case 2: Nancy, France: Dr. Max Amor • • • 83 yo M 85% stenosis 5 mm ICA 7 mm CCA Pre-Dilatation x 2: 5. 0 x 20 • 7 x 25 Casper Deployed • Post-Dilatation x 3: 5. 0 x 20 • 10 -20% residual stenosis Caution: The Casper device is not cleared/approved by the U. S. FDA for sale or use in the United States

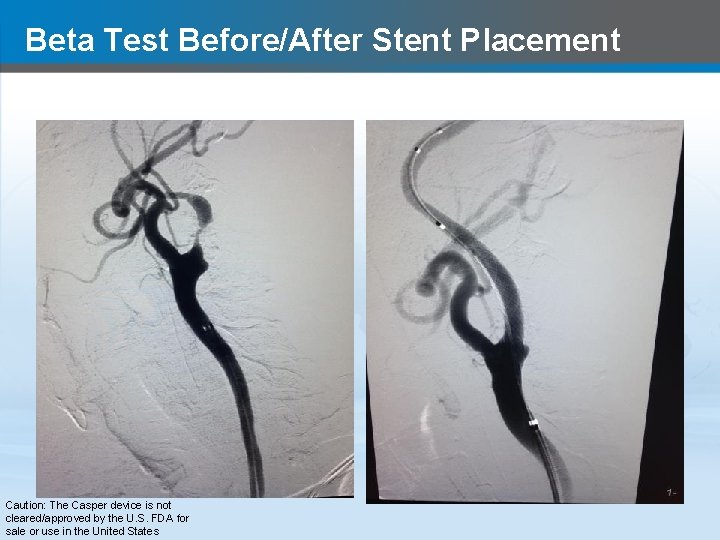
Beta Test Before/After Stent Placement Caution: The Casper device is not cleared/approved by the U. S. FDA for sale or use in the United States

Embolic Protection Device Features • • Ability to Use With Any 0. 014” Wire Concentric Design provides Improved Wall Apposition Equivalent Embolic Capture While Maintaining Flow Minimal Emboli Loss During Recapture Highly Flexible Landing Zone (25 mm from proximal end) All Ni. Ti Construction w/Tantalum Markers Specifications • • • Average Pore Size < 150µ Large size for 4. 5 – 6. 5 mm vessels Small size for 3. 0 – 4. 5 mm vessels Delivery System: 3. 5 Fr. RX Retrieval Catheter: 5 Fr. RX Caution: The Casper device is not cleared/approved by the U. S. FDA for sale or use in the United States