Measurements 1 Measurements A very concrete methods of









- Slides: 20

Measurements 1

Measurements A very concrete methods of dealing with the description and understanding of nature 2

Measurements A very concrete method of dealing with the description and understanding of nature Measurements give credibility to the interpretation of; • Theories • Laws • Principles • Hypothesis 3

Measurements A very concrete way of dealing with the description and understanding of nature Measurements give credibility to the interpretation of: • Theories • Laws • Principles • Hypothesis This credibility is directly related to the accuracy of the measurements 4

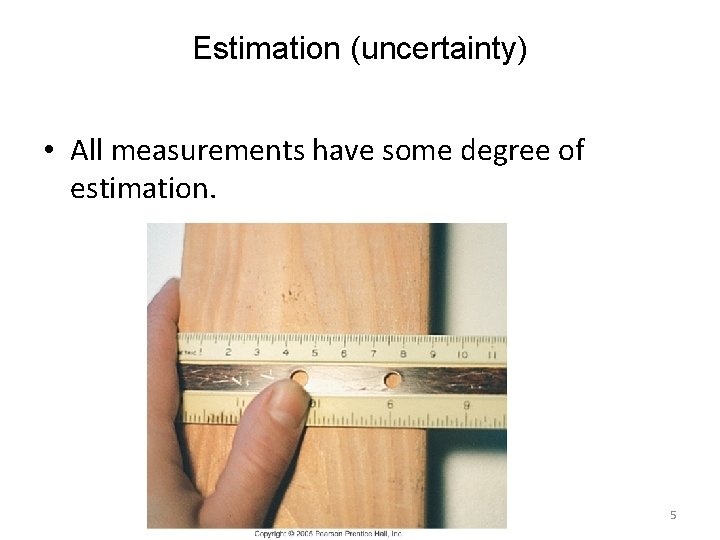
Estimation (uncertainty) • All measurements have some degree of estimation. 5

It would be difficult to measure with an certainty beyond a millimeter 6

The ruler has a limited amount of certainty. Thinner lines could increase the amount of certainty. 7

All measuring devices have a certain amount of uncertainty 8

The uncertainty of a measurement is determined by the a. precision of the measurement and b. accuracy of the measured value 9

Precision verses Accuracy • Precision in its strictest sense refers to the exactness to which the measuring instrument has been manufactured. • If the same measurement is repeated multiple times with the same instrument, will the measurement be the same each time? (repeatability) • smaller units would make the instruments more precise (exact_ • Accuracy is how close the measurement is to the true value • Influenced by : 1. person making the measurements 2. precision of the instrument 10

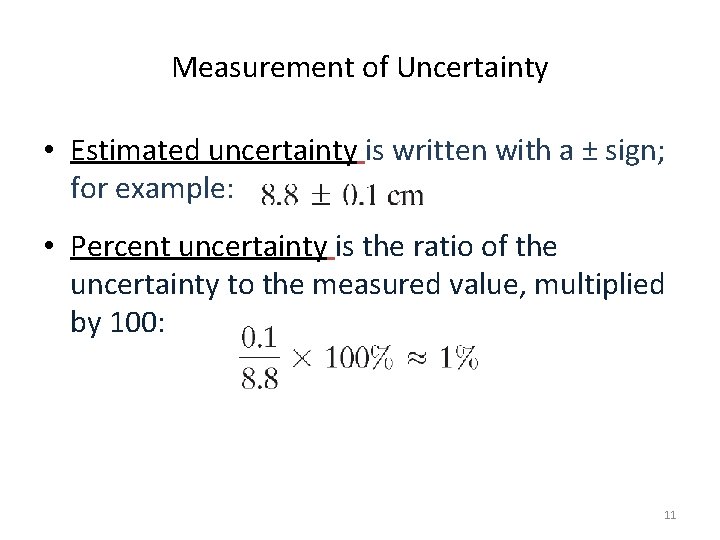
Measurement of Uncertainty • Estimated uncertainty is written with a ± sign; for example: • Percent uncertainty is the ratio of the uncertainty to the measured value, multiplied by 100: 11

12

Significant Figures • The number of significant figures is the number of reliably known digits in a number. It is usually possible to tell the number of significant figures by the way the number is written: • 23. 21 cm has 4 significant figures • 0. 062 cm has 2 significant figures (the initial zeroes don’t count) • 80 km is ambiguous – it could have 1 or 2 significant figures. If it has 3, it should be written 80. 0 km. If it has 2 it should be written in scientific notation 8. 0 x 101 km 13

Significant Figures • When multiplying or dividing numbers, the result has as many significant figures as the number used in the calculation with the fewest significant figures. • Example: 11. 3 cm x 6. 8 cm = 76. 84 cm 2 • 11. 3 cm / 77 cm = 0. 1467 77 cm 2 0. 15 14

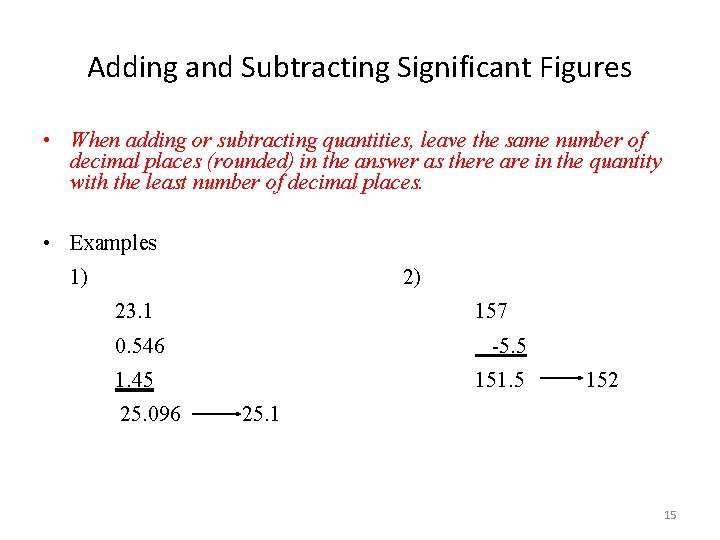
Adding and Subtracting Significant Figures • When adding or subtracting quantities, leave the same number of decimal places (rounded) in the answer as there are in the quantity with the least number of decimal places. • Examples 1) 2) 23. 1 157 0. 546 -5. 5 1. 45 25. 096 151. 5 152 25. 1 15

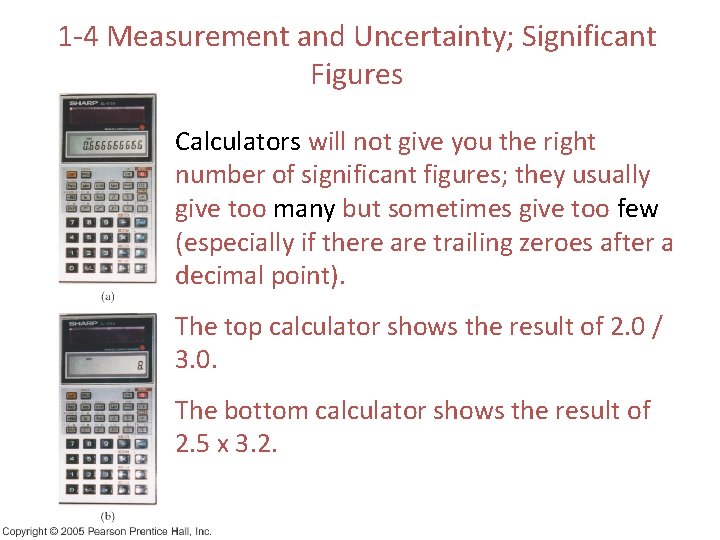
1 -4 Measurement and Uncertainty; Significant Figures Calculators will not give you the right number of significant figures; they usually give too many but sometimes give too few (especially if there are trailing zeroes after a decimal point). The top calculator shows the result of 2. 0 / 3. 0. The bottom calculator shows the result of 2. 5 x 3. 2.

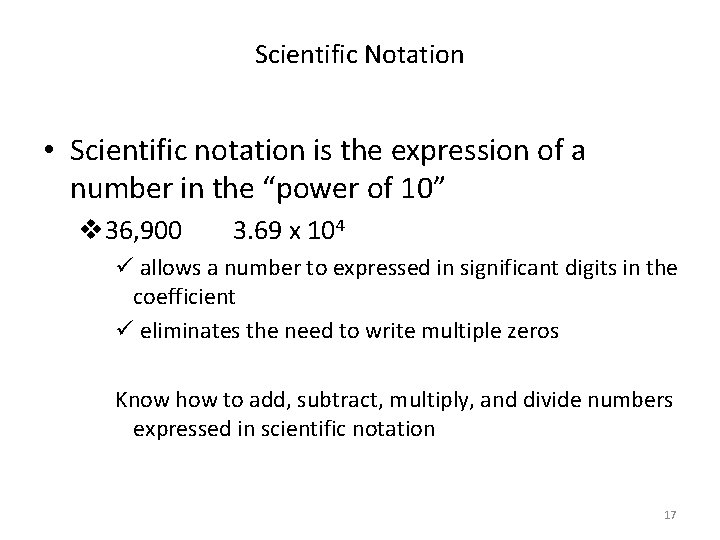
Scientific Notation • Scientific notation is the expression of a number in the “power of 10” v 36, 900 3. 69 x 104 ü allows a number to expressed in significant digits in the coefficient ü eliminates the need to write multiple zeros Know how to add, subtract, multiply, and divide numbers expressed in scientific notation 17

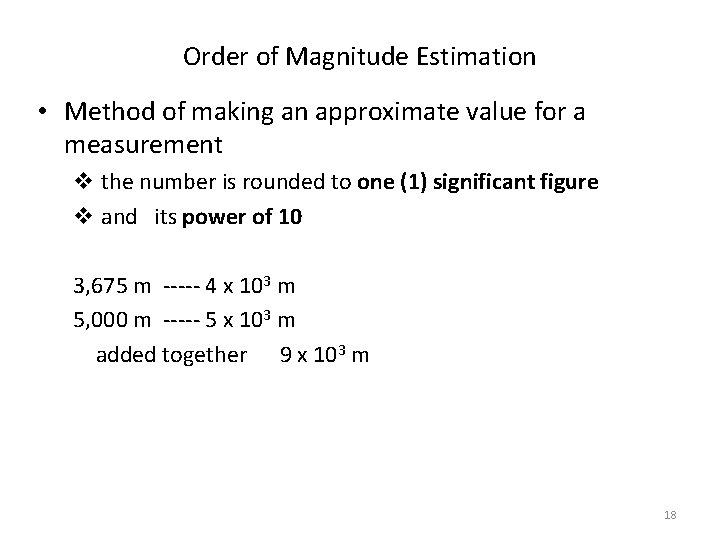
Order of Magnitude Estimation • Method of making an approximate value for a measurement v the number is rounded to one (1) significant figure v and its power of 10 3, 675 m ----- 4 x 103 m 5, 000 m ----- 5 x 103 m added together 9 x 103 m 18

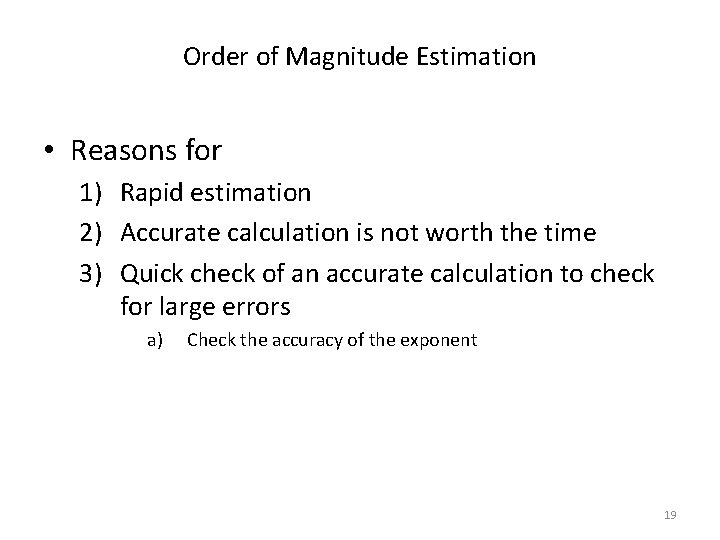
Order of Magnitude Estimation • Reasons for 1) Rapid estimation 2) Accurate calculation is not worth the time 3) Quick check of an accurate calculation to check for large errors a) Check the accuracy of the exponent 19

Order of Magnitude Example • Find the volume (V) of a lake o Lake has o Average depth of 65 m o Surface area of 52, 500 m 2 Volume = area x depth = (7 x 101 m) x (5 x 104 m 2) = 3. 5 x 106 m 3 20
 Concrete semi concrete abstract
Concrete semi concrete abstract Concrete semi concrete abstract
Concrete semi concrete abstract Speedometer
Speedometer Food quantifiers
Food quantifiers Scientific notation rules
Scientific notation rules Very little or very few
Very little or very few Receiving table/area
Receiving table/area Very bad to very good scale
Very bad to very good scale Indirect wax pattern
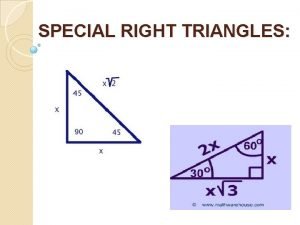
Indirect wax pattern Right triangle formula
Right triangle formula Si unit in science
Si unit in science Kitchen measurements abbreviations
Kitchen measurements abbreviations Measurements
Measurements For adult
For adult Bell measurements for teleportation
Bell measurements for teleportation English linear measurements
English linear measurements It consists of numbers representing counts or measurements.
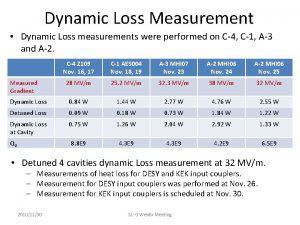
It consists of numbers representing counts or measurements. Measurements were performed
Measurements were performed Measurements in chemistry
Measurements in chemistry Standard deviation of repeated measurements
Standard deviation of repeated measurements Measurements system analysis
Measurements system analysis