Lecture 8 Evapotranspiration 1 Evaporation Processes General Comments







- Slides: 15

Lecture 8 Evapotranspiration (1) Evaporation Processes • General Comments • Physical Characteristics • Free Water Surface (the simplest case) • Approaches to Evaporation Estimation

Evaporation: General Comments • Change of state of water from liquid to gas and thus released to the atmosphere as water vapour • Represents both a loss of mass and energy from a surface • Globally, evaporation is the main control on precipitation and the hydrologic cycle since it releases moisture from the ocean reservoir • Important locally; accounts for up to 75% of water losses from catchments (drainage basins)

Evaporation (Physical Characteristics) 1. Vapour pressure relationship • Vapor pressure at and above evaporating surface • Vapor pressure depends on relative humidity and temperature • Saturation vapor pressure (es) • Actual vapor pressure (ea) 2. Latent heat ( E) • Transfer of latent heat from the evaporating body into the air • Reduces the surface temperature • Latent heat of vaporization: = 2. 47 MJ kg-1 at 10ºC

Evaporation (Physical Characteristics) 3. Sensible heat (H) • Heat energy that we can feel (sense) • Upward rate of sensible-heat exchange by turbulent transfer • Depends on the vertical gradients of wind speed and temperature 4. Bowen ratio • A ratio of sensible-heat exchange to latent-heat exchange

Free Water Surfaces (Simplest Case) Evaporation represents the net flux of water molecules from a surface to the atmosphere. It requires 3 basic conditions 1. Energy Input 2. Vapour Pressure Gradient 3. Exchange Mechanism (Wind)

Required conditions for evaporation 1. Energy Input • Increases temperature of the water • Controls the number of molecules with sufficient energy to break free of the liquid mass (i. e. , latent heat of vaporization) because: (1) Water is a polar molecule (2) Molecules align along the surface (3) Excess kinetic energy required to overcome barrier

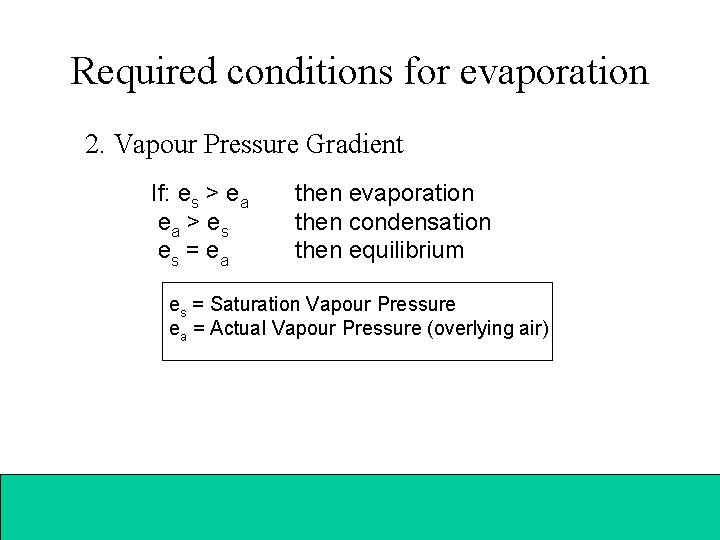
Required conditions for evaporation 2. Vapour Pressure Gradient If: es > ea ea > es es = ea then evaporation then condensation then equilibrium es = Saturation Vapour Pressure ea = Actual Vapour Pressure (overlying air)

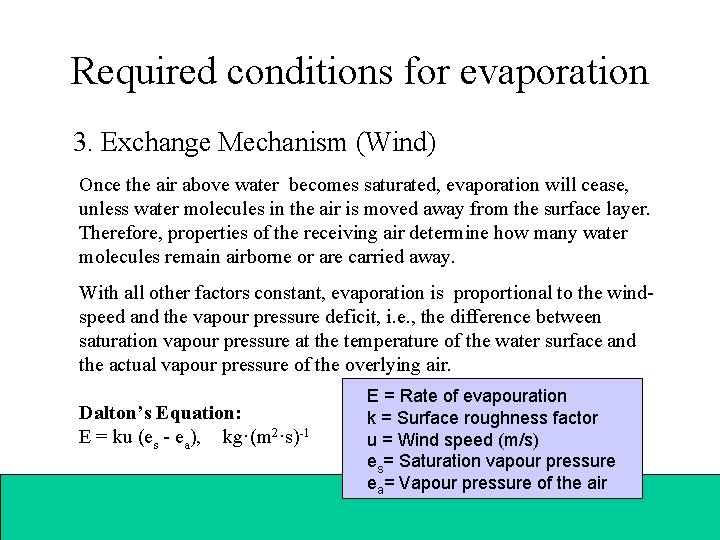
Required conditions for evaporation 3. Exchange Mechanism (Wind) Once the air above water becomes saturated, evaporation will cease, unless water molecules in the air is moved away from the surface layer. Therefore, properties of the receiving air determine how many water molecules remain airborne or are carried away. With all other factors constant, evaporation is proportional to the windspeed and the vapour pressure deficit, i. e. , the difference between saturation vapour pressure at the temperature of the water surface and the actual vapour pressure of the overlying air. Dalton’s Equation: E = ku (es - ea), kg·(m 2·s)-1 E = Rate of evapouration k = Surface roughness factor u = Wind speed (m/s) es= Saturation vapour pressure ea= Vapour pressure of the air

Approaches to Evaporation Studies 1. 2. 3. 4. Water Balance Approach Mass-Transfer Approach Energy Balance Approach Combined Approach

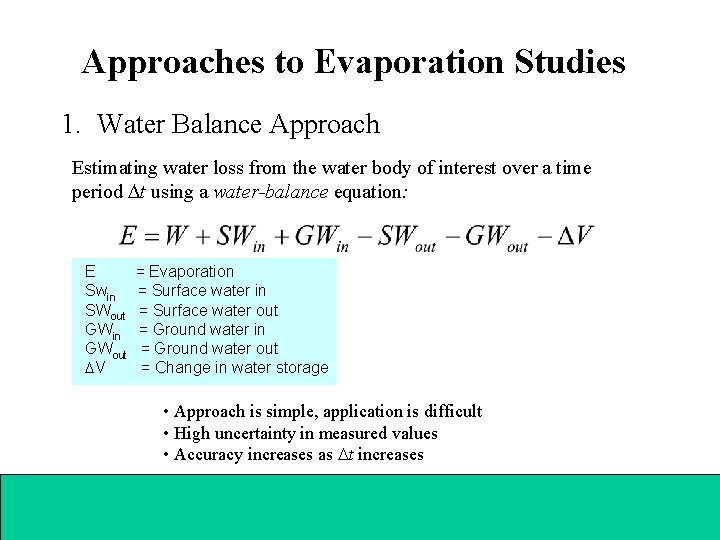
Approaches to Evaporation Studies 1. Water Balance Approach Estimating water loss from the water body of interest over a time period t using a water-balance equation: E = Evaporation Swin = Surface water in SWout = Surface water out GWin = Ground water in GWout = Ground water out V = Change in water storage • Approach is simple, application is difficult • High uncertainty in measured values • Accuracy increases as t increases

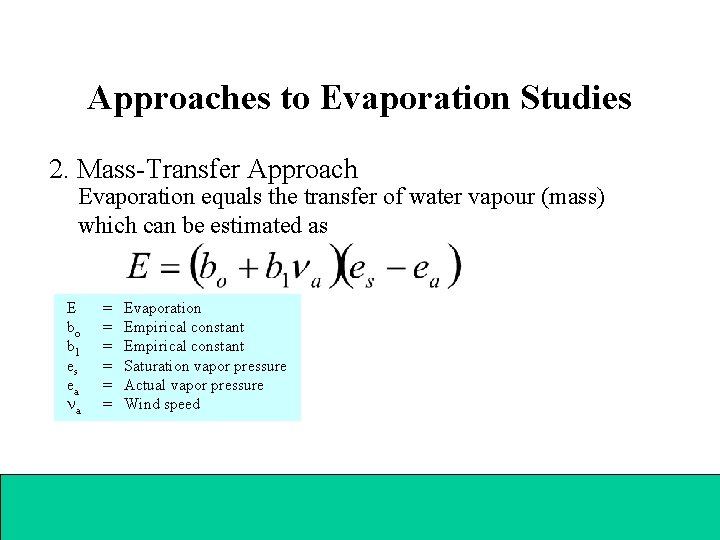
Approaches to Evaporation Studies 2. Mass-Transfer Approach Evaporation equals the transfer of water vapour (mass) which can be estimated as E bo b 1 es ea a = Evaporation = Empirical constant = Saturation vapor pressure = Actual vapor pressure = Wind speed

Approaches to Evaporation Studies 3. Energy Balance Approach Latent heat of vaporization balances energy inputs and outputs for a given period (Electromagnetic radiation) Q* Qh Qg Qe = K - K + L - L = K (1 - ) + L* = Net radiation = Sensible heat flux (surface – atmosphere) = Sensible heat flux (surface – subsurface) = Latent heat flux (surface – atmosphere)

Approaches to Evaporation Studies 3. Energy Balance Approach E K L G H A w Q t w w = = = = = Evaporation Net short-wave radiation input Net long-wave radiation input Net output via conduction to ground Net output of sensible heat to atmosphere Net input associated with in/outflows of water Change in heat storage per unit area Change in time Density of water Latent heat of vaporization

Points to note for the energy balance approach Empirical constants depend on height of wind speed and vapor pressure measurements Difficult to obtain representative measurements of wind speed and vapor pressure from shore-based observation stations Influenced by atmospheric instability – free convection Turbulent transport of water vapor (and heat) away from the surface even in the absence of wind Upward diffusion of water vapour from the evaporating surface “Drying power” of overlying air (humidity) Evaporation is highest when: warm, dry conditions (large saturation deficit), i. e. , es = high, ea = low Diffusion controlled by concentration gradient

Approaches to Evaporation Studies 4. Combined Approach Attempts to consider all factors influencing evaporation. To be studied in next lecture