J TPS Why did Edward Nortons skin start















- Slides: 18



J – TPS • Why did Edward Norton’s skin start to burn off when Brad Pitt put the powder on his skin? • What was happening at the chemical level?

Today’s Learning Targets • 6. 2 – I can describe what an acid and base are using the idea of hydrogen ion donation and hydrogen ion accepting. • 6. 3 – I can characterize a compound as either a strong acid/base or weak acid/based on how much it dissociates.

Arrhenius Acids/Bases • An Arrhenius acid gives a H+ to a solution in an acid/base reaction – An Arrhenius acid will have a H in its chemical compound (e. g. HCl, HNO 3, H 2 SO 4, etc. ) • An Arrhenius base gives an OH- to a solution in an acid/base reaction – An Arrhenius base will have an OH in its chemical compound (e. g. Na. OH, KOH, Ca(OH)2, etc. ).

Bronsted-Lowry Acids/Bases • Refined Arrhenius’ theory of acids/bases • Bronsted-Lowry Acid – Donate (give) a H+ to solution • Bronsted-Lowry Acid – Accept (take) a H+ from solution. • All Arrhenius acids/bases are Bronsted-Lowry acids/bases, but not all Bronsted-Lowry acids/bases are Arrhenius acids/bases.

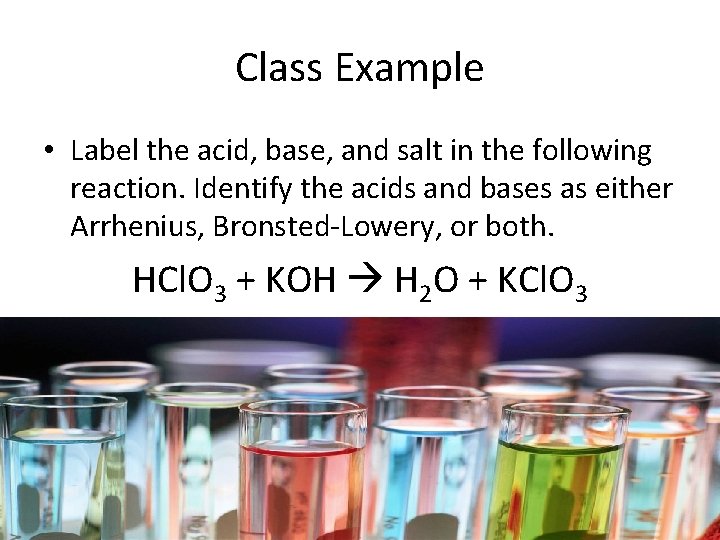
Class Example • Label the acid, base, and salt in the following reaction. Identify the acids and bases as either Arrhenius, Bronsted-Lowery, or both. HCl. O 3 + KOH H 2 O + KCl. O 3

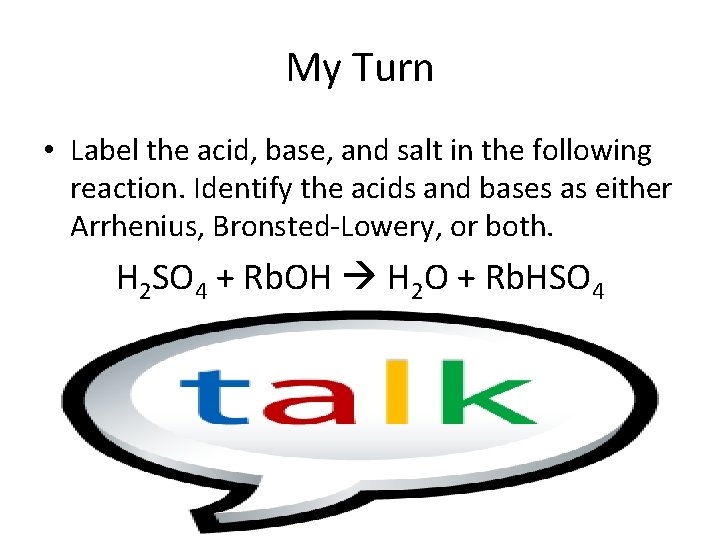
My Turn • Label the acid, base, and salt in the following reaction. Identify the acids and bases as either Arrhenius, Bronsted-Lowery, or both. H 2 SO 4 + Rb. OH H 2 O + Rb. HSO 4

Strength of Acids/Bases • Acids/Bases can be classified as strong or weak based on how much it disassociates. • Disassociate – To break apart • Acids/Bases that completely dissociate are called strong acids/bases • Acids/Bases that only partially dissociate are called weak acids/bases – A solution of weak acid/base has some in the acid/base form and some in the broken apart form.

Strong Acids • • • HI HBr HCl. O 4 HCl. O 3 H 2 SO 4 You must memorize these!

Strong Bases • • Ca(OH)2 Sr(OH)2 Ba(OH)2 Na. OH KOH Rb. OH Cs. OH You must memorize these!

ALL ACIDS/BASES THAT ARE NOT ON THIS LIST ARE WEAK ACIDS/BASES

Summarize

Acid/Base Simulator • With a partner of your choice, take out a computer and the handout you picked up on the way in. • Complete the acid/base dissociation activity

Work Time Complete the worksheet you picked up when you came in.

Learning Log Assessment Rate yourself 1 – 4 on LTs 6. 1, 6. 2, and 6. 3

Exit Slip 1. What are two properties of an acid? 2. What is an Arrhenius acid? 3. What makes something a strong or weak base?

Learning Log Assessment Using your exit slip score, re-rate yourself on LTs 6. 1, 6. 2, and 6. 3